Squamous cell carcinoma of head and neck: what internists should know
Article information
Abstract
Squamous cell carcinoma of head and neck (SCCHN) is a group of cancer arising from mucosal surfaces of the head and neck. Optimal management of SCCHN requires a multidisciplinary team of surgical oncologists, radiation oncologists, medical oncologists, nutritionist, and speech-language pathologists, due to the complexity of anatomical structure and importance of functional outcome. Human papilloma virus (HPV)-related SCCHN represents a distinct subset from HPV negative SCCHN which is associated with carcinogen exposure such as cigarette smoking, betel nut use and alcohol. HPV related SCCHN responds better to concurrent chemoradiation and has better overall prognosis, compared to HPV negative SCCHN. Radiation therapy has been introduced to the treatment of SCCHN, administered concurrently with systemic chemotherapy for locoregional SCCHN, as well as a palliative measure for recurrent and/or metastatic (R/M) SCCHN. Recently, immune checkpoint inhibitors have been shown to improve overall survival in R/M-SCCHN and have been incorporated into the standard of care. Combination approaches with immune therapy and targeted therapy for biomarker enriched population based on genomics are being actively investigated and will shape the future of SCCHN treatment.
INTRODUCTION
Squamous cell carcinoma of head and neck (SCCHN) comprises a heterogeneous group of epithelial neoplasms that arise from upper aerodigestive tract [1]. Almost 65,000 people are estimated to have been affected by SCCHN in the United States in 2019 [2]. In South Korea, 3,309 new cases of cancer in the lip, oral cavity, and pharynx were diagnosed, and 1,170 patients died from the disease in 2015 [3]. Treatment outcomes of SCCHN patients have improved over the past decades: 5-year survival rate of SCCHN in South Korea had jumped from 41.1% to 64.5% in the past 2 decades [3]. This improvement is partly attributed to advance surgical and radiation techniques and better supportive care, but there also has been a major shift in patient characteristics. Historically, smoking and alcohol use were the major etiologic factors of SCCHN with sporadic cases caused by Betel nut chewing or genetic predisposition, such as Fanconi anemia [4-6]. However, over the past decade, human papilloma virus (HPV) has emerged as a pathogen that causes a distinct group of SCCHN, especially oropharyngeal squamous cell carcinoma (SCC) [7]. HPV-related SCCHN affects younger people and has very different genomic features compared to HPV negative SCCHN. After appropriate treatments, HPV-related SCCHN carries significantly better prognosis both in locally advanced disease and in recurrent or metastatic disease [8,9]. HPV-related SCCHN is predicted to reflect changes in sexual practice, although recent implementation of vaccination program against high risk HPV strains may reverse the trend.
HPV RELATED SCCHN
Epidemiology
In 2010, prevalence of oral HPV infection in the United States was estimated to be 6.9% and HPV16 oral infection was estimated to be 1% [10]. In another analysis, prevalence of high risk HPV oral infection was estimated to be significantly higher in males than females (7.3% vs. 1.4%) and males with same-sex partner(s) had even higher infection rate [11]. Among more than 150 HPV serotypes, HPV16 accounts for the majority of HPV associated SCCHN [12] and it primarily affects oropharynx by infecting lymphoid epithelium [13]. The proportion of HPV-positive disease among oropharyngeal cancer has recently increased over time regardless of sex and race, whereas the overall prevalence of SCCHN has remained stable or slightly decreased. In South Korea, prevalence of HPV varied widely depending on the disease site, ranging from 5.3% to 14.5% in oral cavity and from 23.5% to 73.1% in oropharynx [14].
Compared to HPV-negative SCCHN, HPV related SCCHN predominantly affects people with younger age, Caucasian race, and a relatively higher socioeconomic status [8,15]. Association with tobacco, alcohol use, and poor dentition is not very strong in HPV related SCCHN. Instead, there is a positive relationship with history of marijuana use [12]. As the viral infection is sexually transmitted, patients with HPV-positive SCCHN tend to have more lifetime sexual partners and more likelihood of having experience of oral-genital or oralanal contact [15,16]. In a recent cross-sectional study, prophylactic HPV vaccination has shown to be associated with significantly lower risk of having high risk HPV oral infection [17], which raises hope that population wide HPV vaccination program may end the epidemic of HPV related SCCHN in near future.
Biology
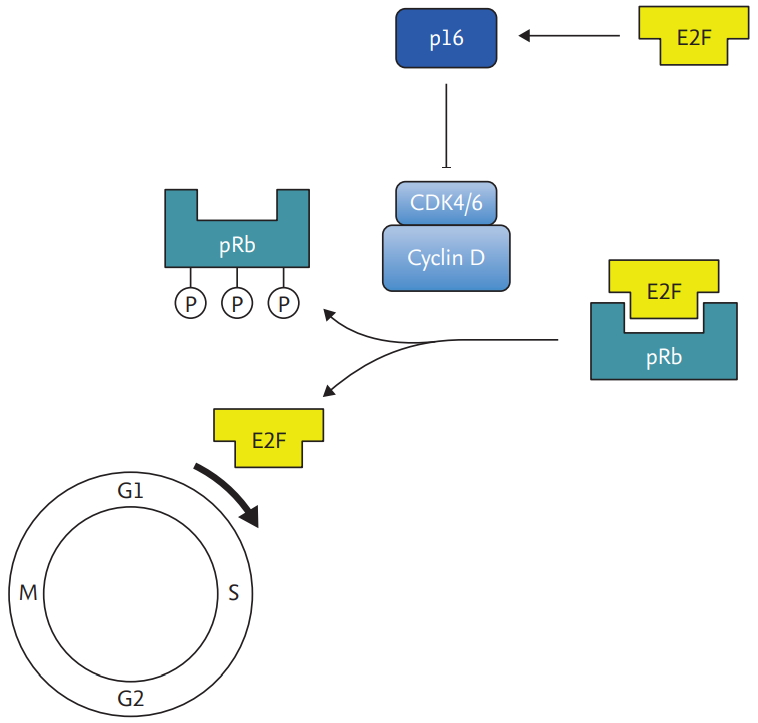
Once transmitted through close contact, HPV invades into micro-wounds of mucosa, and infects the cells in the basal layer of squamous epithelium. E6 and E7 oncoproteins from early phase of HPV life cycle inactivate two key cell cycle regulators, p53 and pRb [18]. E6 binds with E6-associated protein that has ubiquitin ligase activity, which degrades p53, a key tumor suppressor. On the other hand, E7 competitively binds to pRb, releasing transcription factor E2F, which migrates to nucleus and triggers transcription of several genes involved in cell cycle progression and inhibition of apoptosis (Fig. 1) [19]. Both E6 and E7 oncoproteins consequently conduce to abrogation of cell cycle checkpoints, proliferation of cells, and thus, amplification of viral genome. Cells that are persistently infected with HPV continue to propagate, uncoupled from differentiation. These uninhibited cell divisions are prone to accumulated DNA damages and chromosomal abnormalities, which increase oncogenic potential. Although most of the HPV infected cells are removed by immune response in 1 to 2 years, failure to clear the HPV infection results in development of cancer in decades [20,21].
HPV detection for diagnosis
Release of E2F mediated by E7 increases expression p16, which is used as a surrogate biomarker for HPV related oropharyngeal cancer (Fig. 2) [22]. Immunohistochemistry (IHC) for p16 in oropharyngeal SCCs have high sensitivity and specificity and can be reliably used to diagnose HPV related SCCHN [23]. For non-oropharyngeal SCCs, prevalence of HPV related SCCHN is much lower which makes p16 IHC unreliable. HPV DNA detection by polymerase chain reaction has been regarded as the gold standard, but recently direct visualization using RNA probe (RNA in situ hybridization) has been shown to be highly sensitive and specific in detecting transcriptionally active HPV in oropharyngeal cancers [24].
Genomics
Comprehensive genomic analysis of SCCHN in The Cancer Genome Atlas (TCGA) demonstrated significant contrasts in the distribution of genomic alterations between HPV-positive and negative SCCHN [25]. HPV-positive SCCHN harbored frequent alterations of PIK3CA and TNF receptor-associated factor 3 (TRAF3), whereas mutations or copy number variations of TP53, cyclin-dependent kinase inhibitor 2A (CDKN2A), MYC, and cyclin D1 (CCND1) predominantly affected HPV-negative SCCHN. TRAF3, frequently affected in HPV-positive diseases, plays a critical role in innate immune defense, and cells lacking TRAF3 has impaired interferon response against viral infection [26]. Focal amplifications of receptor tyrosine kinase, such as EGFR, ERBB2, and fibroblast growth factor receptor 1 (FGFR1), favored HPV-negative disease. The most common genomic alteration in HPV-positive SCCHN was a helical domain mutation of PIK3CA which encodes a subunit of class 1 phosphoinositide 3-kinase (PI3K) [27]. PI3K receives a signal from cell surface receptors, such as EGFR, and attaches a phosphate group to the inositol head of phosphatidylinositol 4,5-bisphosphate (PIP2) in the cell membrane, converting it to phosphatidylinositol 3,4,5-triphosphate (PIP3) [28]. PIP3, in turn, sends down the signal of cellular growth through the cascade of Akt/protein kinase B and mammalian target of rapamycin (mTOR) [29]. In the TCGA data, mutations in the PI3KCA were concentrated in the frequent ‘hotspot’ mutations (E542, E545 in the helical domains) in HPV-positive SCCHN, whereas mutations were more spread out in HPV-negative diseases [25,27,30,31].
Clinical features
Patients with HPV-positive SCCHN tend to have less smaller primary tumors, larger cystic lymph node, better performance status, and better prognosis compared to patients with HPV negative SCCHN [8,32]. In vitro studies also have shown that HPV infected cancer cells are more prone to apoptosis in response to DNA damaging agents [33,34]. In a prospective cohort study of oropharyngeal and laryngeal SCC, patients with HPV-positive disease had longer progression free survival (PFS) and overall survival (OS), and the improved survival persisted after careful adjustment for age, tumor stage, and Eastern Cooperative Oncology Group (ECOG) performance status. Considering the sensitivity to treatment and better prognosis, patients with HPV-positive SCCHN may benefit from less toxic, de-intensified treatment strategy, which should be a focus of future studies. Differences of disease characteristics between HPV-positive and negative SCCHN are summarized in Table 1.
CURRENT STANDARD OF TREATMENT
Locally advanced SCCHN
About 40% of the patients with SCCHN initially present with early stage, localized disease [35]. These patients can be effectively treated with a single modality therapy, either curative intent surgery or definitive radiation, depending on tumor location and the institution’s experience. While surgery has been the traditional treatment for these cancers, definitive radiation can safely replace surgery with comparable oncologic outcomes, and achieve better functional outcomes in larynx, hypopharynx, or oropharynx.
For patients with locally advanced SCCHN, multi-modality therapy involving at least two different modalities are required. If upfront surgery is offered, post-operative (adjuvant) radiation therapy should be considered with or without concurrent chemotherapy. Definitive radiotherapy with concurrent chemotherapy is also widely used, but it is not preferred upfront approach for oral cavity SCCHN for inferior oncologic outcomes and higher complication rates [36]. Recent developments in minimally invasive surgery, such as transoral robotic surgery (TORS), has enabled surgeons to gain easier access to certain areas of oropharynx such as base of tongue, which traditionally required an extensive procedure involving split of mandible. TORS has been widely adopted for treatment of HPV positive oropharyngeal SCC, as patients are expected liver longer and more motivated to avoid late consequences from radiotherapy and/or chemotherapy. A small randomized phase 2 trial comparing upfront TORS versus definitive (chemo)radiotherapy demonstrated that upfront radiotherapy was slightly better in terms of swallowing related quality of life (QOL) measure, but the difference was not clinically meaningful [37]. More clinical trials comparing definitive radiation and TORS are on-going in early stage oropharyngeal SCCs.
Chemotherapy alters DNA binding and creates reactive free radicals, enhancing the cytotoxic effect of radiation [38]. Besides, chemotherapy inhibits DNA repair and recovery from potentially lethal or sub-lethal damages in between radiation sessions [39]. Several clinical trials have confirmed that concomitant administration of chemoradiation significantly prolongs survival for locally advanced SCCHN, compared to radiation alone, or sequential administration of chemotherapy and radiation [40-45]. Concurrent chemoradiation was superior to radiation alone in postoperative settings as well for patients who have high risk pathologic features such as positive margins and/or extranodal extension [46,47]. Based on these findings, concurrent chemoradiation has been widely adopted for the treatment of locally advanced SCCHN.
Induction chemotherapy prior to definitive radiation or chemoradiation has been contemplated for long time to achieve immediate response and decrease risk of distant metastasis. However, a series of clinical trials of induction chemotherapy compared to upfront concurrent chemoradiation failed to demonstrate superior survival, even in the patients with high risk patients with bulky tumor or lymph node [48-50]. Furthermore, toxicities from induction chemotherapy may delay or interrupt definitive chemoradiation. Neoadjuvant chemotherapy prior to definitive surgery has been evaluated in a large phase 3 randomized trial in oral cavity cancers, but failed to demonstrate any overall or disease-free survival benefit [51]. Therefore, induction or neoadjuvant chemotherapy is not considered a standard treatment for locally advanced SCCHN. Nonetheless, for patients with bulky, symptomatic primary or nodal disease, induction chemotherapy can be started if the initiation of chemoradiation is delayed.
Cetuximab, an anti-EGFR antibody, has been used as a concurrent therapy with radiation. Cetuximab plus radiation demonstrated OS and PFS benefits compared to radiation alone for patients with locally advanced SCCHN [52], and cetuximab was viewed as a way to decrease treatment-related toxicity in definitive concurrent chemoradiation, especially for HPV related SCCHN patients who have excellent prognosis. Two large randomized clinical trials evaluated concurrent cetuximab in comparison to concurrent cisplatin with definitive radiotherapy in locoregionally advanced SCCHN with intent of demonstrating non-inferiority of outcomes. However, to the contrary of the expectations, concurrent cetuximab with radiation was shown to have worse OS and PFS in both studies [53,54]. For HPV negative SCCHN, it is not clear whether cisplatin would be still superior to cetuximab as a concurrent treatment, but cisplatin remains to be the standard concurrent systemic agent.
Recurrent and/or metastatic SCCHN
Although majority of patients with SCCHN present with locally advanced disease, approximately 30% to 40% of patients develop recurrence even with intensive multimodality treatment [45]. For selected patients with locoregional recurrence, surgical salvage or re-irradiation can be attempted, but it has not been very successful [55]. Recent retrospective case series suggests that surgical salvage for locoregional and distant failure in oropharyngeal SCC can be associated with improved OS in both HPV-positive and -negative patients, although the benefit in oligometastatic disease was largely limited to HPV positive disease [56]. Even after recurrent or metastatic disease, patients with HPV positive SCCHN have longer OS compared to the ones with HPV negative SCCHN [9,57]. However, median disease free survival after salvage surgery remains poor for both HPV-positive and HPV-negative patients [58]. Metastatic and/or recurrent disease not amenable for surgical salvage can be treated with systemic therapy which includes cytotoxic chemotherapy, targeted therapy and immune checkpoint inhibitors.
Combination of platinum, fluorouracil, and cetuximab (EXTREME regimen), which was shown to prolong OS to 10.1 months, used to be the standard of care for the 1st line treatment of recurrent and/or metastatic SCCHN [59]. Cetuximab has a modest single agent activity for SCCHN when used after failing prior platinum-based chemotherapy [60]. Generally, conventional systemic treatment options are limited as responses are generally short-lived and not durable with high toxicity toll. Immune checkpoint inhibitors bind to molecules that mediates immune tolerance such as programmed death 1 (PD1) receptors which are located on the membrane of T cells, B cells, and natural killer cells. When bound with ligands on tumor cell surface, these immune checkpoint molecules inhibit cancer cell apoptosis, and down-regulate cytotoxic T cell function [61,62]. Immune checkpoint inhibitors re-stimulate the immune function to target cancer cells.
Anti-PD1 antibodies have been evaluated in recurrent/metastatic (RM)-SCCHN patients in the 2nd line setting after progression on platinum containing chemotherapy. Pembrolizumab demonstrated overall response rate of 16% in a single arm study, which led to accelerated approval of pembrolizumab by the U.S. Food and Drug Administration. This trial included even patients with no programmed death-ligand 1 (PD-L1) expression, and 12% of these patients still demonstrated objective responses. Response rates were comparable between HPV-positive and negative groups [63]. Nivolumab was evaluated in a phase 3 randomized clinical trial, compared to a single agent therapy of investigators’ choice. This study demonstrated significant OS benefit with 1-year survival rate of 36% versus 16.6% [64]. Of note, patients who received nivolumab had better QOL outcome in terms of social function, fatigue, dyspnea, pain, and appetite. Median time to deterioration in several domains of QOL was significantly longer with nivolumab than with standard chemotherapy [65]. Nivolumab is currently approved by the U.S. Food and Drug Administration for the treatment of recurrent or metastatic SCCHN after progression on platinum-based chemotherapy.
More recently, pembrolizumab with or without chemotherapy was evaluated in the 1st line RM-SCCHN in comparison to the EXTREME regimen in a randomized, phase 3 clinical trial [66]. The study demonstrated that pembrolizumab alone has significant OS benefit in patients with PD-L1 expressing tumor (defined by combined positive score > 1) and combination of pembrolizumab and chemotherapy has significant OS benefit in all patients. Pembrolizumab with or without chemotherapy was approved based on this study for the 1st line use in RM-SCCHN. Of note, overall response rate of pembrolizumab alone was significantly lower than conventional chemotherapy, while that of pembrolizumab plus chemotherapy was comparable to the chemotherapy. This implies that pembrolizumab and chemotherapy combination might be more beneficial for or patients with bulky disease who would benefit from rapid reduction of tumor burden, regardless of PD-L1 expression status.
Approval of immune checkpoint inhibitors in the 1st line treatment of RM-SCCHN limits options for 2nd line treatment. A study suggests chemotherapy would be still active and viable option for patients who received immune checkpoint inhibitors in the first line setting. Objective response was seen in 37.5% of 16 patients who received cetuximab after anti-PD1 failure and 44.4% of 27 patients who received chemotherapy based regimen [67].
MULTIDISCIPLINARY CARE AND IMPORTANCE OF FUNCTIONAL OUTCOME
SCCHN involves very complex and delicate structures. Mucosal surface of oral cavity, pharynx and larynx covers vital passage of food and air and located very close to other important anatomical areas such as skull base and carotid artery. Oral cavity, pharynx and larynx are responsible for normal breathing, swallowing and speech. Treatment of SCCHN may impact all of these functions and may cause trismus, neck and shoulder dysfunction, vascular complications, dysphagia, xerostomia, dental caries, taste disorder, fatigue, sleep dysfunction, and hypothyroidism [68]. These long-term consequences of treatment can lead to loss of appetite, weight loss and chronic aspiration induced lung damage and feeding tube dependency, which negatively impact patients’ QOL and body image. Long term consequences from treatment of head and neck cancer can vary depending on treatment modality used, thus it is important to consider functional outcome in addition to the oncological outcome when making a treatment decision.
It is important to remember that concurrent chemoradiation was developed as a way to preserve larynx function in patients with laryngeal SCC. Radiation Therapy Oncology Group (RTOG) 91-11 study, which randomized stage III/IV laryngeal cancer patients to the Veterans Affairs (VA) laryngeal study approach (induction chemotherapy then radiation), concurrent chemoradiation (cisplatin), and radiation alone, showed that concurrent chemoradiation was superior to the others in achieving laryngeal preservation and locoregional control [43]. However, there was a substantial risk of overall treatment failure with 25% of the entire study population eventually receiving salvage laryngectomy. Moreover, patients with a high volume T4 disease were excluded in this study leaving 90% of the participants with T2 or T3 tumors. In patients with bulky diseases treated with non-surgical approach, the clinical benefit of sparing already deformed larynx is possibly low because of subsequent aspiration risk [69]. It was also noted in a long-term follow-up that there was a possibility of worse outcome from concurrent chemoradiation as more deaths occurred from causes other than laryngeal cancer [45].
Radiation treatment is the frequent culprit for pharyngeal constrictor muscle damage and intractable aspiration [70]. It has been demonstrated that there is a positive relationship between radiation dose and volume/thickness of pharyngeal constrictor muscle as well as level of acoustic-articular changes in patients with oropharyngeal cancer [71,72]. Toxicities from radiation or chemotherapy often exacerbate voice or swallowing function in an already dysfunctional, cancer-riddled upper aerodigestive tract. Among the patients with oropharyngeal cancer who had been treated with radiation with or without chemotherapy, Penetration Aspiration Scale (PAS) was abnormal in 45% of them, which was also independently associated with pre-treatment swallowing difficulty [73]. Nonetheless, patients with SCCHN have demonstrated coping mechanisms and self-adjustment. In an assessment of voice and swallowing function using the Voice Related Quality of Life (VR-QOL) measure and the list Performance Status Scale for Head and Neck Cancer Patients (PSS-HN), patients with laryngeal cancer who had received organ preservation treatment of concurrent chemoradiation, although, understandably, scored worse than normal subjects, had higher QOL scores than patients with non-cancerous voice disorders, such as vocal fold paralysis or adductor spasmodic dysphonia. Their QOL results were also significantly higher than patients who underwent laryngectomy, especially in terms of understandability of speech. However, swallowing function, dietary restriction, and frequency of eating in public was not better compared to those who had laryngectomy. Factors that were associated with likelihood of eating in public were, instead, longer time duration since the last treatment and lower grade of mucositis [74].
Interestingly, patient’s perception of QOL does not seem to correlate with objectively measured level of swallow dysfunction [75], which provides an important clue in understanding patients’ disease awareness and perception of QOL. Self-reported symptoms by patients should not be solely relied upon when assessing swallowing capacity and nutritional status as they are not accurate indicators of level of morbidity.
With regard to swallowing dysfunction, patients may need a gastric feeding tube for nutritional support during chemoradiation. Feeding tubes can be placed prophylactically prior to initiation of therapy or reactively when patients develops need for nutritional support. A systematic review suggests that prophylactic feeding tube reduces the number of malnourished (> 10% loss of body weight) patients and improves QOL after treatment, but does increase chance for long term feeding tube dependence [76]. A retrospective review of treatment outcome of concurrent platinum-based chemoradiation for advanced laryngeal cancer showed that 7% of the patients required percutaneous gastrostomy, 6% had persistent dysphagia, and 2% had chronic lung aspiration [77].
Salivary gland is sensitive to radiation and can be easily damaged by radiation causing permanent dysfunction [78]. Xerostomia and hyposalivation are common late toxicities of head and neck radiotherapy and can contribute to the development of dental demineralization and caries. Approximately 21% to 24% of SCCHN patients treated with radiation or chemoradiation develops dental caries [79], due to decreased buffering capacity, insufficient calcium and phosphate, proliferation of cariogenic bacteria and diet change [80]. Dental demineralization may lead to rampant dental breakdown and osteonecrosis. Therefore, a comprehensive dental evaluation is essential, and any underlying dental problems associated with poor dental outcome need to be addressed prior to initiation of radiotherapy. Ongoing dental care including maintenance of good oral hygiene and regular supplementation of fluorides is necessary after therapy [81].
SURVIVORSHIP AND SUPPORTIVE CARE
Patients with SCCHN are living longer as novel pharmaceuticals and therapeutic techniques are being developed. Among the patients with HPV-positive disease, 5-year survival rate reached nearly 90% [82]. Patients who previously underwent invasive treatment for SCCHN frequently suffer from long-term functional or esthetic sequelae and bear a concern or fear of cancer recurrence in their daily lives [83]. As a result, they are more vulnerable to psychosocial illnesses, such as depression and anxiety, which negatively impact social and occupational activities. Therefore, SCCHN patients often require close and personalized psychosocial care for a long-term period after the treatment. Comprehensive care for SCCHN survivors also includes assessment and management of late-occurring symptoms, general health promotion, and surveillance of recurrence or second primary cancer. To meet each individual’s needs and requirements, SCCHN survivorship program necessitates multidisciplinary efforts from primary care, speech therapy, physical therapy, social care, psychiatry and oncology subspecialties.
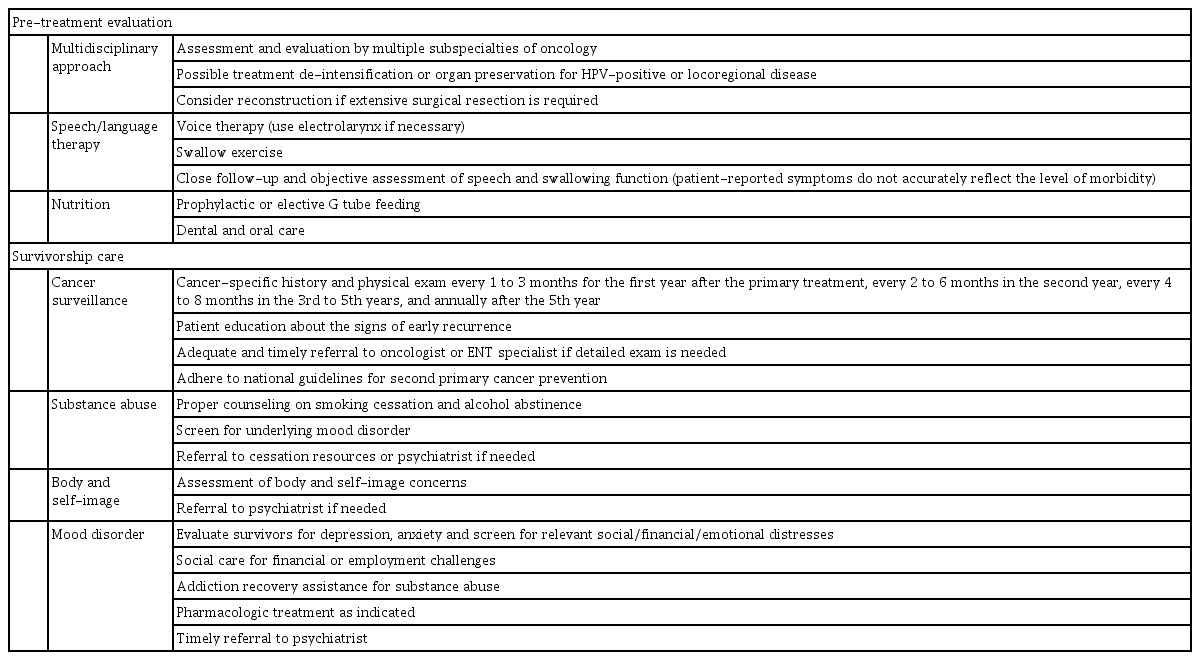
American Cancer Society (ACS) Head and Neck Cancer Survivorship Care Guideline recommends that primary care physician should obtain cancer-related history and physical exam every 1 to 3 months for the 1st year after the primary treatment, every 2 to 6 months in the 2nd year, every 4 to 8 months in the 3rd to 5th years, and annually after the 5th year. Patients should continue follow-up with head and neck surgeons for more detailed and focused exam. Primary care physicians are also responsible for educating survivors about the signs of early recurrence. For early detection of second primary cancer, patients should adhere to national guidelines for general cancer prevention [84].
Smoking and alcohol abuse, which is common among patients with SCCHN, can increase the risk of second primary cancer. However, smoking and alcohol cessation rates after the cancer diagnosis are inadequately low. Among the SCCHN patients with previous or current smoking history at the time of diagnosis, approximately 25% to 35% of them continued to smoke after the treatment [85,86], and the smoking rate was higher after non-surgical treatment compared to surgery [85]. Furthermore, alcohol-dependent behavior became increased 1 year after the SCCHN diagnosis regardless of treatments received [87]. Therefore, it is important to counsel the survivors on smoking cessation and alcohol use.
SCCHN survivors frequently experience dramatic changes in self-perception of body image. This concerns multiple aspects of psychosocial outcome, causing mood disorders and avoidance of social activity. Their social function is more impaired when they feel discouraged to eat or speak in public due to self-consciousness of facial difference. Severe changes in self-perception may also jeopardize employment and financial status [88]. These issues may contribute to high prevalence of mood disorders among SCCHN survivors: 17%, 15%, and 13% of SCCHN patients who were disease free after radiation treatment reported that they are “somewhat depressed” or “extremely depressed” after 1, 3, and 5 years, respectively. In spite of high prevalence of depression at 5 years, none of the patients were receiving anti-depressants or psychotherapy at the time [89]. Direct comparison should be interpreted with caution, but prevalence of depression was 11.6% among the cancer survivors (> 2 years) in general and 10.2% in healthy controls [90]. Managing mood disorder for SCCHN survivors is crucial as depression is negatively correlated with OS and disease recurrence, even after adjusting for other relevant factors [91]. It is incumbent on primary care physicians to evaluate SCCHN survivors for depression or anxiety every three months post-treatment using an appropriate diagnostic tool. Patients should be offered pharmacologic interventions or referral to specialists if indicated. Comprehensive management of mental distress also includes social care for financial and employment challenges, or addiction recovery assistance for substance abuse. Key recommendations for supportive treatment and survivorship care are summarized in Table 2.
CONCLUSIONS
Current researches on head and neck cancer treatment are focused on a few areas. For locoregionally advanced SCCHN, there is an effort to reduce the burden of treatment related toxicities with equivalent outcomes for HPV related disease. The efforts include adoption of induction chemotherapy as a selection tool for patients to receive reduced amount of radiation and use of reduced amount radiation in combination with immune checkpoint inhibitors or chemotherapy. ECOG 1380 study was a phase 2 study which used induction chemotherapy upfront for HPV positive SCCHN. If patients respond to the chemotherapy, they received reduced amount of radiation (54 Gy as opposed to 70 Gy) with concurrent cetuximab. 70% of patients received reduced amount of radiation and their 2-year PFS was 96% [92]. This study suggests that induction chemotherapy can be used as a patient selection tool for treatment de-intensification. For HPV positive disease, NRG HN002 study was a phase 2 study with reduced dose radiation (60 Gy) with or without concurrent cisplatin for favorable risk HPV positive SCCHN. The radiation alone arm did not meet the prespecified 2 year PFS goal of 85%, while the concurrent cisplatin arm did meet the goal [93]. Based on the result, NRG HN005 study is being conducted to compare standard of care (70 Gy plus cisplatin), reduced dose radiation with cisplatin and reduced dose radiation with an immune checkpoint inhibitor, nivolumab (NCT03952585). For HPV negative, locoregionally advanced SCCHN, efforts are focused on intensifying current standard of care to improve the suboptimal outcome. Many clinical trials are investigating addition of immune checkpoint inhibitor to the concurrent chemoradiation (NCT03040999, NCT02952586) or after the concurrent chemoradiation (NCT03452137).
For RM-SCCHN, many researches are focused on enhancing the activity of immune checkpoint inhibitors by adding more agents including HPV vaccine, immune adjuvants, or other immune checkpoint inhibitors. For example, an HPV vaccine, ISA101, demonstrated objective response rate (ORR) of 33% in combination with nivolumab in a phase 2 study [94]. However, these activities need to be interpreted with caution and should be confirmed in larger phase 3 studies and interpreted with caution. Recently, precision oncology-based approaches are gaining more attention in RM-SCCHN. Traditionally, frequent mutations in SCCHN were thought to be non-actionable as most of the genomic alterations were found in tumor suppressor genes such as TP53. However, a small number of SCCHN does harbor distinct oncogenic driver mutations in genes like HRAS and PIK3CA. A recent clinical trial with tipifarnib, a farnesyl-transferase inhibitor which inhibits activity of HRAS, demonstrated 56% ORR in 18 SCCHN patients with HRAS mutations [95], and a larger study is underway to confirm the activity.
There are also research efforts to reduce the toll of toxicities related to treatment. Superoxide dismutase mimetics have been thought to be agents to alleviate radiation related toxicities. A randomized, phase 2 study of GC4419, a superoxide dismutase mimetic, versus placebo, in locoregionally advanced SCCHN, demonstrated that GC4419 does decrease incidence of several oral mucositis [96]. Agents in this class are being under evaluation in larger studies at this time.
Notes
No potential conflict of interest relevant to this article was reported.