The effect of antiplatelet drug on coronary endothelial and microvascular function: comparison with ticagrelor and clopidogrel
Article information
Abstract
Background/Aims
Coronary endothelial and microvascular function play important roles in cardiovascular disease. We aimed to evaluate the effect of ticagrelor on coronary artery function and tested the antiplatelet effect of low dose ticagrelor in East-Asian patients.
Methods
Sixty-one consecutive patients with non-significant coronary disease were included in the study. Initially, patients were randomized in 1:1:1 ratio to receive drugs: ticagrelor 90 mg twice a day (bid; n = 22), ticagrelor 45 mg bid (n = 19) or clopidogrel 75 mg once a day (qd; n = 20) and then divided into two groups (ticagrelor vs clopidogrel) for evaluation of coronary artery function, and three groups for evaluation of antiplatelet function. Endothelial dysfunction was measured by coronary flow reserve (CFR), and changes in the levels of asymmetric dimethylarginine (ADMA), cluster of differentiation (CD) 40 ligand, and P-selectin. Microvascular function was evaluated as index of microvascular resistance (IMR). Platelet reactivity was assessed by VerifyNow P2Y12 assay.
Results
The levels of CFR, ADMA, and CD 40 ligand were not different between the two groups. However, P-selectin was lower in the ticagrelor group compared with clopidogrel group. IMR was significantly lower in the ticagrelor group compared with clopidogrel group (median, 15.0 [interquartile range, 12.0 to 21.0] vs. 47.5 [23.0 to 67.5], p = 0.014). There was significant difference in platelet inhibition among the three groups (ticagrelor 90 mg bid vs. ticagrelor 45 mg bid vs. clopidogrel 75 mg qd; 85.57 ± 47.63 vs. 120.33 ± 51.09 vs. 256.42 ± 55.10, p < 0.001)
Conclusions
It is hypothesized that ticagrelor might ameliorate the coronary microvascular function. When compared with clopidogrel, low dose ticagrelor exhibited satisfactory antiplatelet effect in the present study.
INTRODUCTION
Platelets and associated antiplatelet drugs play an essential role in the pathogenesis of acute coronary syndrome and the treatment of coronary artery diseases (CADs). Over the years antiplatelet treatment has evolved to a great extent and currently several types of antiplatelet drugs are available. Ticagrelor is a potent, novel, direct-acting, and reversibly binding P2Y12 receptor antagonist which was approved for the treatment of patients with acute coronary syndrome [1]. Although ticagrelor and clopidogrel act on same antiplatelet target receptor, ticagrelor significantly reduces the incidence of myocardial infarction, stroke, or death from vascular causes compared with standard treatment with clopidogrel in the Platelet Inhibition and Patient Outcomes trial [2]. These differences in results might not be simply explained based on the antiplatelet effect. Apparently there exists a hypothesis that ticagrelor might exhibit pleiotropic effects beyond those related to potential antiplatelet effect and that these might be relevant to its clinical efficacy.
Recent studies showed that ticagrelor treatment positively impacts endothelial function and inhibit the release of platelets’ stored inflammatory mediators [3,4]. There is little data about its effect on coronary microvascular dysfunction.
In addition, Asian patients are believed to be more susceptible to bleeding, when compared with non-Asian patients [5]. Clinical pharmacology studies have reported higher levels of inhibition for platelet aggregation in Asian patients compared with Whites [6,7]. But, there is no data about the appropriate dose of ticagrelor, a potent antiplatelet drug, for Asian patients.
So, we aimed to evaluate the effect of ticagrelor on coronary endothelial and microvascular function in patients with CAD in comparison with effects of clopidogrel. We also tested the antiplatelet effect of low dose ticagrelor in East-Asian patients.
METHODS
Study design
This study was a single-center, open-label, and randomized controlled trial that compared the effect of ticagrelor and clopidogrel on coronary endothelial and microvascular function suggestive of worse clinical outcomes. In addition, various antiplatelet doses were evaluated for identification of appropriate effects in East-Asian patients. From May 2016 to January 2017, patients who had chest pain were enrolled from outpatient clinic at Konkuk University Chungju Hospital. Initially, patients were randomized in a 1 : 1 : 1 ratio to receive drugs for at least 7 days: (1) ticagrelor 180 mg loading and then 90 mg twice daily (n = 22), (2) ticagrelor 180 mg loading and then 45 mg twice daily (n = 19), or (3) clopidogrel 300 mg loading and then 75 mg once a day (qd; n = 20). Subsequently, the patients were divided into two groups (ticagrelor vs clopidogrel) for evaluation of endothelial and microvascular function, and into three groups (ticagrelor 90 mg twice a day [bid] vs. ticagrelor 45 mg bid vs. clopidogrel 75 mg qd) for evaluation of antiplatelet function, respectively. Patients underwent laboratory testing, including assessment of endothelial function, inflammation markers, antiplatelet assay, and coronary angiography with coronary physiologic assessment (Fig. 1).
Eligibility criteria included stable angina patients older than 18 years and younger than 85 years, with non-significant (visually less than 70% stenosis and more than the value of 0.8 on fractional flow reserve) CAD. Exclusion criteria included bypass graft lesion; previous percutaneous coronary intervention (PCI) for the target vessel; heart failure; ejection fraction less than 45%; myocardial infarction at the time of study enrollment; patients who required treatment with positive inotropic agents other than digoxin during the study; patients with cerebrovascular accident within 6 months before entry into the study; and significant endocrine, hepatic or renal disorders. We obtained study approval from the Institutional Review Board of Konkuk University Chungju Hospital (IRB No. KUCH 2016-02-009) and all subjects provided written informed consent.
Endothelial function and inflammation marker
Before coronary angiography, quantitative measurements of high-sensitivity C-reactive protein levels with routine laboratory tests were performed. Whole blood was centrifuged for 15 minutes at 3,000 rpm, aliquoted, and immediately frozen at 80°C until analysis. The markers of platelet activation and associated endothelial function (soluble cluster of differentiation [CD] 40 ligand and soluble P-selectin) were assessed using a quantitative enzyme-linked immunosorbent assay technique (Bender Med Systems GmbH, Vienna, Austria) according to the manufacturer’s instructions. Additionally, the plasma concentration of asymmetric dimethylarginine (ADMA) was measured for assessment of endothelial function as previously described [8].
Coronary physiologic assessment
To assess coronary endothelial and microvascular function, we measured the index of microvascular resistance (IMR) and coronary flow reserve (CFR). Intracoronary pressure parameters in each patient were measured using previously described principles [9].
With commercially available software (Abbott Vascular Inc., Santa Clara, CA, USA), the shaft of the pressure wire can act as a proximal thermistor, sensor near the tip of the wire simultaneously measured the pressure and temperature acting as a distal thermistor. Thermo-dilution technique was used to check the transit time of room temperature when saline was injected into coronary arteries. 3 mL aliquots of room temperature saline was administered to the coronary artery, and the resting mean transit time (rTmn) was measured. Steady state of maximal hyperemia was induced by intravenous infusion of adenosine (140 μg/kg/min). Three additional 3 mL aliquots of room temperature saline were injected, and the hyperemic mean transit time (hTmn) was measured. The simultaneous measurements of mean aortic pressure (Pa, by guiding catheter) and mean distal coronary pressure (Pd, by pressure wire) were also obtained during the resting state and maximal hyperemic state (Fig. 2).

Calculation of index of microvascular resistance and coronary flow reserve. IMR, index of microvascular resistance; Pd, distal pressure; Tmn, mean transit time; CFR, coronary flow reserve; FFR, fractional flow reserve.
The CFR was calculated by dividing the resting hTmn by the rTmn. The IMR was defined as the simultaneously measured distal coronary pressure divided by the inverse of the hTmn (Fig. 2).
Platelet function test
Blood samples at baseline were collected via the sheath in the catheterization laboratory prior to the procedure. P2Y12 reaction units (PRU) were measured using the VerifyNow assay (Accumetrics, San Diego, CA, USA). In brief, the VerifyNow assay is a whole blood, cartridge-based, optical detection system designed to measure platelet aggregation [10]. Within the cartridge of the VerifyNow P2Y12 assay is a channel in which inhibition of the adenosine diphosphate (ADP) P2Y12 receptor is measured. This channel contains ADP as a platelet agonist and prostaglandin E1 as a suppressor of intracellular-free calcium levels to reduce non-specific contribution of ADP binding to P2Y12 receptors, and the numerical results are expressed as PRU.
Statistical methods
All the statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). For continuous variables, differences between the groups were evaluated by unpaired t test or Mann–Whitney rank-sum test. For discrete variables, differences were expressed as counts and percentages and analyzed with chi-squared or Fisher’s exact test between the groups as appropriate. A two-tailed p value of 0.05 was considered to be statistically significant. Results are reported as mean ± standard deviation (SD), median (interquartile range [IQR]), or numbers (percentage).
RESULTS
Baseline clinical characteristics
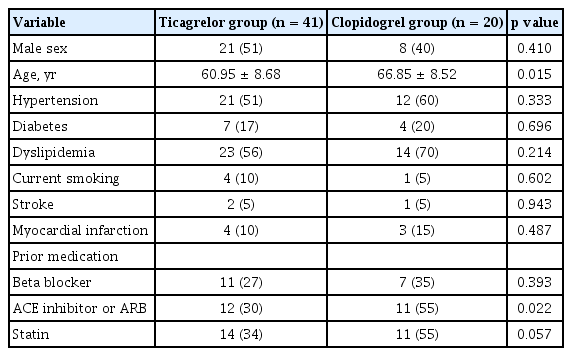
The baseline clinical characteristics of patients are shown in Table 1. The prevalence of diabetes mellitus, hypertension, hyperlipidemia and myocardial infarction was similar in both the groups except for age. There was no significant difference in medication except for the renin-angiotensin-aldosterone system inhibitor. There was no significant difference in total cholesterol, triglyceride, and low and high density lipoprotein cholesterol between these two groups except for the creatinine level. However, estimated glomerular filtration rate was similar in the two groups (Table 2).
Endothelial and inflammation makers between ticagrelor and clopidogrel groups
Soluble P-selectin was significantly lower in the ticagrelor group compared with clopidogrel group (70.68 [IQR, 55.20 to 80.30] vs. 99.65 [IQR, 70.90 to 134.78], p = 0.019). However, there were no differences in the levels of soluble CD 40 ligand (2,788.16 [IQR, 1,914.31 to 4,276.39] vs. 3,145.95 [IQR, 2,407.01 to 4,745.52], p = 0.325) and ADMA (0.60 [IQR, 0.53 to 0.67] vs. 0.62 [IQR, 0.58 to 0.81], p = 0.294) between the two groups (Fig. 3). In the subgroup analysis, there were no differences in the levels of CD 40 ligand, P-selectin and ADMA among the three groups (ticagrelor 90 mg bid vs. ticagrelor 45 mg bid vs. clopidogrel 75 mg qd; p = 0.077 for P-selectin, p = 0.574 for CD 40 ligand, p = 0.752 for ADMA).
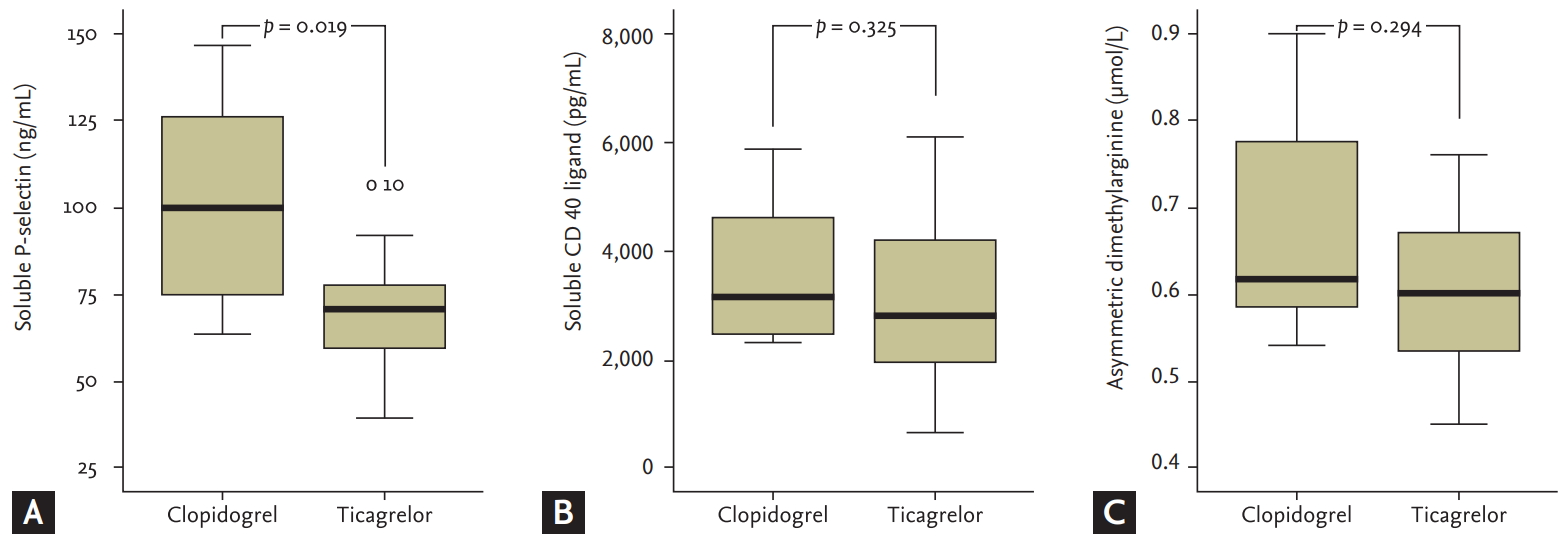
Comparison (A) P-selectin, (B) soluble cluster of differentiation (CD) 40 ligand, and (C) asymmetric dimethylarginine between ticagrelor group and clopidogrel group. Soluble P-selectin was significantly lower in the ticagrelor group compared with clopidogrel group, but there were no differences in the soluble CD 40 ligand and asymmetric dimethylarginine between two groups.
Coronary angiographic characteristics and coronary physiologic assessment
The angiographic parameters, fractional flow reserve (FFR), CFR and IMR are shown in Table 3. Angiographic characteristics were comparable in both the groups except for the reference diameter. The diameter of the patient group receiving ticagrelor was larger compared with the patients receiving clopidogrel. There was no difference in the FFR between both the groups. Fig. 4 illustrates comparison of CFR and IMR between the two groups. CFR was not significantly different in both the groups (2.0 [IQR, 0.7 to 5.5] vs.1.85 [IQR, 1.13 to 3.60], p = 0.731). However, IMR was significantly lower in the patient group receiving ticagrelor than clopidogrel group (15.0 [IQR, 12.00 to 21.00] vs. 47.5 [IQR, 20.75 to 67.50], p = 0.014). IMR was widely distributed with higher level in the clopidogrel group compared with the ticagrelor group.
Platelet function test among the three groups
There were significant differences among the three groups with regards to platelet function (ticagrelor 90 mg bid, 85.57 ± 47.63 vs. ticagrelor 45 mg bid, 120.33 ± 51.09 vs. clopidogrel 75 mg qd, 256.42 ± 55.10, p < 0.001) (Fig. 5).

Comparison antiplatelet function among ticagrelor 90 mg twice a day (bid), ticagrelor 45 mg bid and clopidogrel group. Although ticagrelor 90 mg bid group had the strongest antiplatelet function among the three groups, platelet inhibition of ticagrelor 45 mg bid group was also significantly stronger than that of clopidogrel group. PRU, P2Y12 reaction unit.
Although the ticagrelor 90 mg bid group had the strongest antiplatelet function among the three groups, platelet inhibition of ticagrelor 45 mg bid group was also significantly stronger than that of clopidogrel group suggestive of sufficient dose for East-Asian patients (Fig. 5).
In the association between platelet function and adhesion molecule, soluble P-selectin was significantly correlated with antiplatelet function (r = 0.529, p = 0.014) (Fig. 6).
DISCUSSION
To the best of our knowledge, this is first study to simultaneously compare coronary endothelial and microvascular functions between patients with ticagrelor and those with clopidogrel. In our study, the administration of ticagrelor significantly improved coronary microvascular function whereas no effect was observed in endothelial function.
There were several reports that antiplatelet drugs could affect endothelial or vascular function. Clopidogrel or prasugrel improves endothelial nitric oxide bioavailability and diminishes biomarkers of oxidant stress and inflammation in patients with CAD, suggesting that beyond inhibition of platelet aggregation, adenosine phosphate receptor blockade may also have promising vasoprotective effects [11,12]. A recent study showed that ticagrelor induced significant increase in circulating endothelial progenitor cells, contributing to improved arterial endothelial function in diabetic non–ST-segment elevation acute coronary syndrome patients compared with prasugrel [13].
However, the association among endothelial marker, microvascular function and antiplatelet drugs in patients with CAD has not been studied. In the present study, we demonstrated that ticagrelor was considerably associated with coronary microvascular function based on assessment by IMR but not endothelial function by CFR or biomarker. This result is inconsistent compared with the results of previous studies [11,13-16]. These observations might be explained as follows: we included patients with relatively stable disease state and insignificant stenosis. Apparently, our absolute hs-CRP level was relatively low compared with other studies even though atherosclerosis is generally an inflammatory process. P-selectin and soluble CD 40 ligands are from different expression cells but similar clinical properties. P-selectin is a single chain glycoprotein of 140 kDa expressed in mostly platelets and endothelial cells but soluble CD 40 ligand, which is shed from stimulated lymphocytes and is actively released after platelet stimulation [17,18]. Consequently soluble CD 40 ligand was considered as an independent risk marker of cardiovascular events in acute coronary syndrome [19,20]. In contrast, increased level of soluble P-selectin has been reported in a wide variety of acute and chronic cardiovascular conditions [21,22]. Therefore, we assumed that P-selectin was significantly lower by potent antiplatelet drug in our patient population with stable state compared to CD 40 ligand.
Consideration the relationship between CFR and IMR, our results showed discordance between CFR and IMR even though CFR reflects coronary microvascular function. The previous study also showed about 30% discordance between CFR and IMR in high FFR patients and described the clinical relevance of IMR and CFR is unclear [23]. In addition, CFR can be highly variable according to the hemodynamic dependence of basal flow. So, it is difficult to define and maintain true resting conditions during CFR measurement. In contrast, IMR was known that it provides a more reproducible assessment of the microcirculation than CFR [9].
It is well known that the presence and the degree of microvascular and endothelial dysfunctions are related to the risk of subsequent cardiovascular events [24,25]. In our study, the coronary microvascular function was better in the ticagrelor group compared with clopidogrel group. The coronary microcirculation to regulate myocardial perfusion is regulated myocardial metabolic activity and endothelium-dependent vasodilation effectively and efficiently [26]. In our study, IMR and P-selectin levels were lower in the ticagrelor group. ADMA and CD 40 ligand were comparable between the two groups. Although further studies are needed for elucidating the exact mechanism [27], ticagrelor is suggested as an antiplatelet drug, as it might affect microvascular function through platelet-endothelial dependent pathway but not anti-inflammatory pathway.
We also evaluated platelet function for a low dose of ticagrelor for Asian patients, who are believed to be more susceptible to bleeding. In the results, even the low dose ticagrelor showed significantly stronger antiplatelet function compared with standard clopidogrel dose similar to other studies [28,29]. It is proposed that low dose of ticagrelor impart a more benefit-risk balance than ticagrelor 90 mg bid or clopidogrel for East-Asian patients.
This study has several limitations. First, in the present study, the population studied was small, and further investigations with a larger population are required in future studies. Second, the antiplatelet medication interval was relatively short. Most of the patients underwent treatment for 7 days. It is hypothesized that ticagrelor could demonstrate better results for endothelial markers if administered longer period. However, Gao et al. [30] evaluated the endothelial function with ticagrelor administration for 7 days. In another study, microvascular function was evaluated after just 180 mg loading dose [31]. Third, drug cross-over was not designed. Under conditions of cross-over for antiplatelet drugs, the effect of each drug on microvascular and endothelial functions could be clarified. Lastly, reference diameter was bigger in ticagrelor group compared with clopidogrel group and we measured IMR for intermediate stenotic artery. However, minimal microvascular resistance does not change with epicardial stenosis severity [32,33]. In contrast, another study showed IMR significantly decreased after PCI [34]. In our study, cases with visually significant stenosis were excluded.
The results reveal that ticagrelor might ameliorate the coronary microvascular function. This effect could explain the reason for ticagrelor being superior to clopidogrel in randomized controlled trial. In addition, when compared with clopidogrel, low dose ticagrelor exhibited sufficient antiplatelet effect. Evaluation of antiplatelet effect on coronary endothelial function is necessitated in the future studies. Low dose ticagrelor is proposed as an alternative antiplatelet treatment instead of full dose ticagrelor in East-Asian patients.
KEY MESSAGE
1. Ticagrelor might ameliorate coronary microvascular function.
2. Low dose ticagrelor has sufficient antiplatelet effect in East-Asian patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2016.