Impact of subsequent chemotherapy on the survival of elderly patients with extensive stage small cell lung cancer
Article information
Abstract
Background/Aims
The prognosis of small cell lung cancer (SCLC) is still poor because of rapid recurrence, despite good response to initial chemotherapy. Additionally, patients’ old ages and comorbidities are often obstacles that make it difficult to apply subsequent treatment after initial treatment. This retrospective study analyzed the correlation of post-progression survival (PPS) with overall survival (OS), and prognostic factors including comorbidities to figure out impact of subsequent chemotherapy on OS in elderly extensive disease SCLC.
Methods
We analyzed 101 patients of age 65 years or older who were recently diagnosed with extensive disease SCLC (ED-SCLC) in Korea University Medical Center between January 1995 and December 2015. The degree of comorbidity was scored using simplified comorbidity score (SCS). Correlation between PPS, progression-free survival (PFS) and OS was analyzed using a Pearson correlation coefficient. Cox proportional hazards regression was employed to examine the influence of clinical variables on survival.
Results
Median age of patients was 71 years old (range, 65 to 83). Median OS was 8.7 months (range, 0.3 to 42.7). PPS was a reliable factor on OS than PFS (R2 = 0.852, p < 0.001). Prognostic factors associated with improved survival were SCS < 9, administration > 4 cycles of first line chemotherapy and subsequent second line chemotherapy.
Conclusions
PPS was more correlated with OS than PFS in elderly patients with ED-SCLC. The most important prognostic factors for PPS and OS included SCS and second line chemotherapy. Patients receiving subsequent treatment had increased OS regardless of degree of comorbidity.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths worldwide [1] as well as in Korea. Over 20 percent of cancer patients die from lung cancer [2]. Small cell lung cancer (SCLC) accounts for approximately 15 to 20 percent of lung cancers in Korea. This is one of the most aggressive types of cancer characterized by rapid progression, high frequency of metastasis, and high initial response rate to chemotherapy [3]. Patients’ old age and comorbidities further confound treatment procedures. Despite good response to initial chemotherapy, the prognosis is poor because of rapid recurrence. The median survival for limited disease SCLC (LD-SCLC) is 12 to 17 months and for extensive disease SCLC (ED-SCLC) is 7 to 9 months [4,5]. This malignancy is highly correlated with tobacco smoking [3]. Majority of patients are heavy smokers with more than 40 pack-years. For this reason, SCLC is commonly accompanied by comorbidities associated with smoking, such as chronic obstructive pulmonary disease (COPD) and cardiovascular disease. Although most medical oncologists believe that extensive malignant tumors should be treated with palliative chemotherapy, many physicians are hesitant to treat these patients because of their old age, impaired lung function, or cardiovascular comorbidities. Similar reasons affect the decision to conduct subsequent chemotherapy after initial treatment.
In order to confirm the need for subsequent treatment, it is necessary to evaluate the impact of post-progression survival (PPS) on overall survival (OS). Previous studies evaluating PPS reported that it was strongly associated with OS and subsequent treatment after first line chemotherapy might influence OS in many cancer types, including lung cancer [6-8].
It is also important to ensure that subsequent treatment is available to SCLC patients. In elderly patients, an objective assessment of the comorbidities is essential since they can affect treatment and OS. Simplified comorbidity score (SCS), which is one of the scoring systems using weighted items to quantify a patient’s comorbidity, has been validated as an independent prognostic factor in the treatment of non-small cell lung cancer (NSCLC) [9,10]. A subsequent study has suggested that the SCS might also be used as a prognostic factor for patients with SCLC [11]. However, the National Comprehensive Cancer Network (NCCN) guidelines still recommend performance status (PS) score as a criterion for deciding chemotherapy in SCLC patients [12].
Therefore, we investigated the correlation of PPS with OS; the factors that influence PPS and OS, including SCS; and the factors that determine the need for subsequent treatment in elderly patients with ED-SCLC.
METHODS
Patients
A retrospective study was performed after obtaining approval from the Institutional Review Board (IRB) of Korea University Ansan Hospital (IRB No.: 2017AS0106). Written informed consent by the patients was waived due to a retrospective nature of our study. We included patients of age 65 years or older who were recently diagnosed with ED-SCLC in Korea University Medical Center Guro and Ansan (1,054 and 710 beds, respectively) tertiary teaching hospital in South Korea. The patients were selected based on electronic medical records between January 1995 and December 2015. All patients were diagnosed on the basis of histologic examination of the malignant lesion. Staging was done based on the Veterans Administration Lung Study Group system [13]. Extensive disease status was confirmed by imaging studies including computed tomography (CT) and positron emission tomography (PET). SCS was applied as a prognostic factor before chemotherapy. It is composed of seven comorbid conditions: (1) tobacco consumption (weighting 7; total consumption is more than 100 cigarettes); (2) diabetes mellitus (weighting 5; under medication control); (3) renal insufficiency (weighting 4; creatinine clearance < 60 mL/min by Cockroft-Gault formula); (4) respiratory condition (weighting 1; history of tuberculosis, pleural effusion, pneumonia, asthma, pulmonary embolism, chronic hypoxemia < 60 mmHg, and COPD with a forced expiratory volume in 1 second < 1.5 L); (5) cardiovascular conditions (weighting 1; congestive heart failure, ischemic cardiopathy, severe valvular heart disease, arrhythmia under chronic treatment, history of cardiovascular disease, and peripheral vascular disease); (6) neoplastic conditions (weighting 1; previous cancer history); and (7) alcoholism (weighting 1; daily alcohol consumption of > 80 g of alcohol in men and > 40 g in women) [9]. Before chemotherapy, all patients were carefully checked by physical examination, laboratory test, pulmonary function test, and diagnostic image test including chest X-ray, CT, and PET. During chemotherapy, response was evaluated every two cycles as possible. In every visit, physical examination, routine laboratory test, chest X-ray were checked by physicians. After discontinuing primary treatment for any reason, follow-up was performed at intervals of several months, including imaging studies. After the progress of the disease was confirmed, second line treatment was carried out at the discretion of the clinician.
Statistical analysis
Progression-free survival (PFS) was defined as the period from the date of diagnosis until the date of progression or death from any cause. PPS was defined as the period between disease progression and death from any cause [14]. OS was defined as the period from the date of diagnosis until the date of death from any cause or last follow-up date. Correlation between PPS, PFS and OS was analyzed using a Pearson correlation coefficient. In bivariate correlation analysis, R2 means correlation coefficient which show degree of correlation between two factors. This coefficient close to one suggests highly correlated with one another. Logistic regression was used to determine which covariates have influenced prognosis. Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI). Univariate analysis was performed to determine associations between patients and individual factors. Multivariable analysis was performed between the factors having statistically significant correlation with patients and OS. The factors of p value ≤ 0.05 were considered to be statistically significant. Survival analysis was also conducted using the Kaplan-Meier method and compared with log-rank test. IBM SPSS Statistics version 20.0 for Windows (IBM Co., Armonk, NY, USA) was used for all statistical analyses in this study.
RESULTS
Patient characteristics
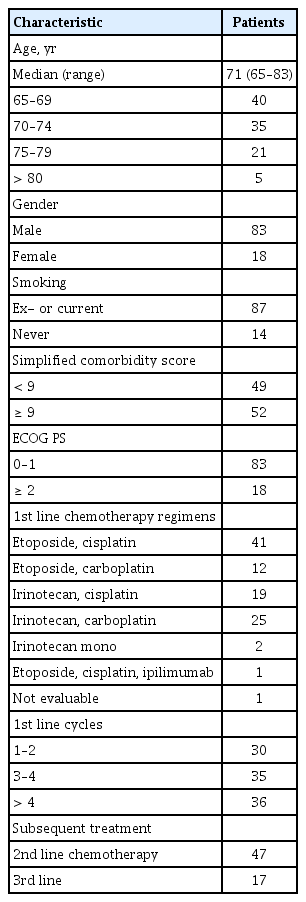
A total of 101 patients in the age group of 65 years or older were diagnosed with ED-SCLC. Table 1 summarizes the main characteristics of patients. Median age of the patients was 71 years old (range, 65 to 83). Most of the patients were male (n = 83, 82%) and 18 were female (18%). About 86% of the patients were current or ex-smokers. All patients were confirmed to have ED-SCLC at the time of diagnosis. SCS was divided into 9 points as references [9-11]. Half of the patients (n = 52) got more than 9 points in SCS. ECOG performance scores were generally good as 0 to 1 (76%). Half of the patients were treated with etoposide and cisplatin or carboplatin (52%). The other regimens included irinotecan and platinum. Two-thirds of the patients were treated with less than four cycles of first line chemotherapy. Thirty-six patients received more than four cycles of first line chemotherapy. Treatment response was assessed by the Response Evaluation Criteria in Solid Tumor (RECIST 1.1) [15]. After the first line chemotherapy, 47 patients who had confirmed progression were treated with second line chemotherapy. The most common regimen used for second line chemotherapy was irinotecan with platinum or alone. In addition, etoposide, belotecan, and topotecan were also used. Other patients were unable to undergo second line chemotherapy due to sudden deterioration of the systemic condition, refusal of treatment, interruption of visit, or death. Only 17 patients had undergone third line chemotherapy (Table 1).
Treatment and survival
Median OS of the patients in this study was 8.7 months and median PPS after first line chemotherapy was 3.1 months. We also analyzed PPS with OS and PFS. We found that PPS was more reliable than PFS in reflecting OS (Fig. 1). Correlation coefficient (R2) was 0.852 between PPS and OS (p < 0.001). R2 between PFS and OS was 0.426 (p < 0.001).

Correlation between overall survival and (A) post-progression survival, (B) progression-free survival using a Pearson’s correlation coefficient. Overall survival and post-progression survival (R2 = 0.852). Overall survival and progression-free survival (R2 = 0.426). R2 , Pearson’ correlation coefficient.
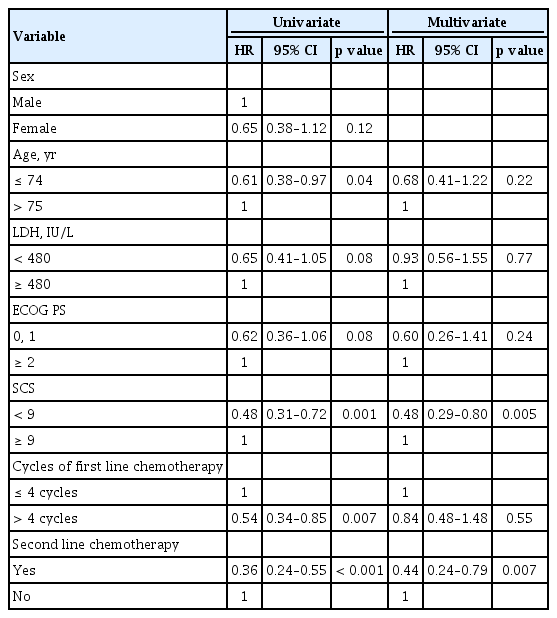
Prognostic factors with favorable PPS were analyzed in this study. In univariate analysis, age < 75, SCS < 9, administration of > 4 cycles of first line chemotherapy, and undergoing second line chemotherapy were associated with improved survival. In multivariate analysis, SCS (< 9 vs. ≥ 9; HR, 0.48; 95% CI, 0.29 to 0.80, p = 0.005) and undergoing second line chemotherapy (HR, 0.44; 95% CI, 0.24 to 0.79, p = 0.007) were defined as significant prognostic factors for PPS (Table 2). In univariate analysis, prognostic factors associated with improved OS were ECOG PS < 2, SCS < 9, administration of > 4 cycles of first line chemotherapy, and undergoing second line chemotherapy. In multivariate analysis, SCS (< 9 vs. ≥ 9; HR, 0.44; 95% CI, 0.28 to 0.69, p < 0.001), administration of first line chemotherapy (> 4 cycles vs. ≤ 4 cycles; HR, 0.50; 95% CI, 0.30 to 0.81, p = 0.005), and undergoing second line chemotherapy (HR, 0.56; 95% CI, 0.34 to 0.91, p = 0.02) were defined as significant prognostic factors for OS (Table 3).
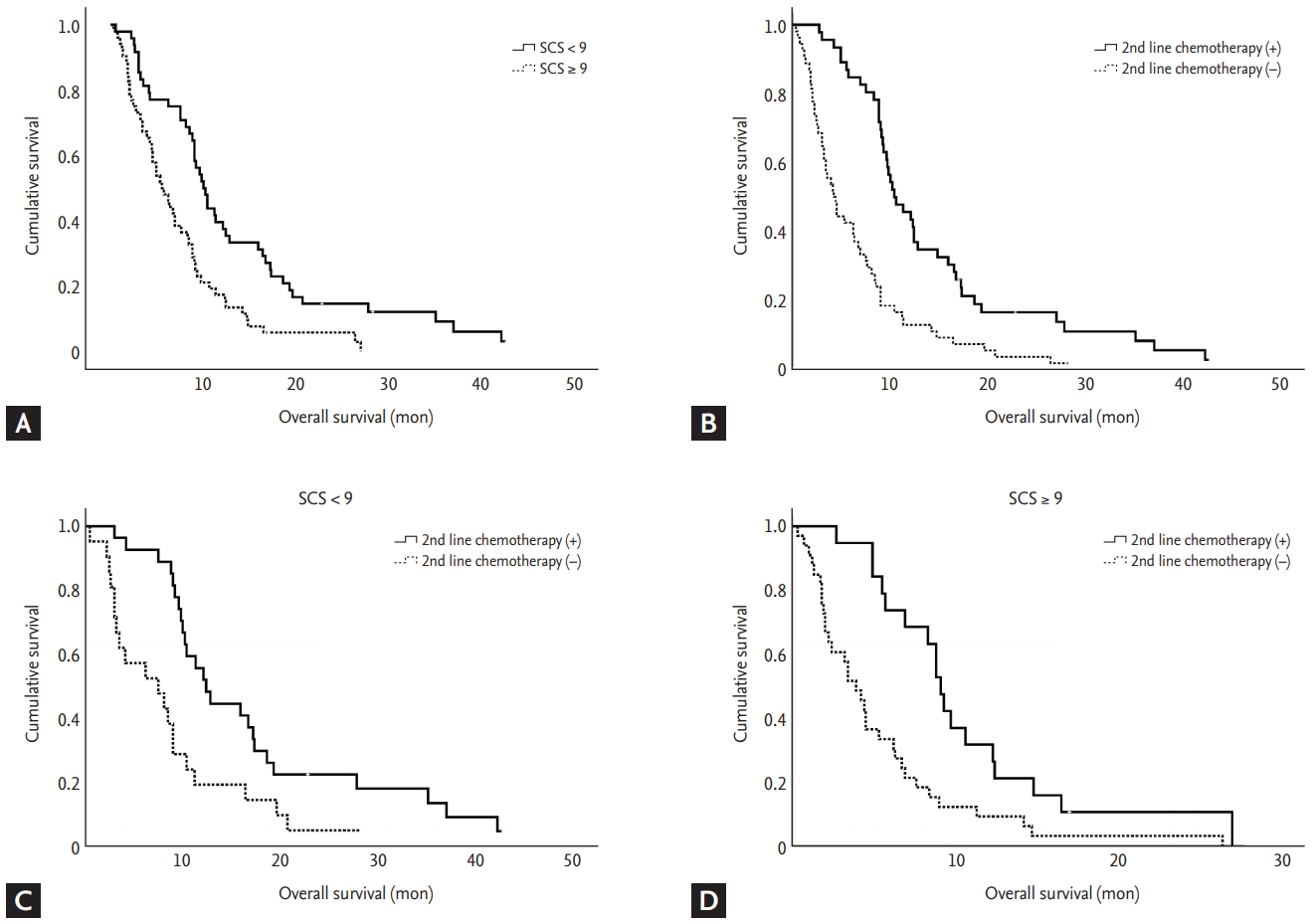
Fig. 2 describes the Kaplan-Meier curves for OS for each group categorized by SCS and/or second line chemotherapy. Patients with SCS < 9 showed significantly longer OS than those with SCS ≥ 9 (Fig. 2A). Similar result was found in the group of patients who underwent second line chemotherapy (Fig. 2B). The patients who received second line chemotherapy showed favorable OS in both groups of SCS < 9 and ≥ 9 (Fig. 2C and 2D).
DISCUSSION
In this study, PPS showed stronger association with OS than with PFS in elderly patients with ED-SCLC. This finding suggests that proper management, after failure of first line chemotherapy, prolonged OS. In fact, second line chemotherapy, which is the most powerful method for management, was found to be one of the most important factors determining OS as well as PPS.
In clinical trial settings, PFS has been the most common endpoint for OS and is known as the surrogate marker for OS [16,17]. Although the measurement of PFS is easier and more convenient, the disadvantage is that the effect of subsequent lines of therapy on the OS can be overlooked [18]. PPS was more important than PFS as there are various, novel options for therapy after first line treatment. Previous studies have reported that the number of active compounds available after initial treatment has increased and the treatment with these could influence the OS in breast, ovarian, and colorectal cancers [7,8,19]. Similarly, there have been several reports that the development of drugs such as pemetrexed or EGFR TKI has made PPS more important than PFS for initial treatment in NSCLC [6,20,21]. Even in SCLC, which has fewer options for subsequent treatment, there has been evidence that PPS has more relevance to OS [22-24], which is consistent with our results. According to the studies mentioned above, the independent prognostic factors for PPS were the response to second line chemotherapy, the number of subsequent treatments, and other clinical stages or PS [25,26]. The present study included few patients who received more than third line of chemotherapy, so we could not analyze the number of subsequent treatments. However, we confirmed that second line chemotherapy was an independent prognostic factor in both PPS and OS.
Recently, immunotherapy, a potentially new treatment approach, was suggested, for the first time in decades, in SCLC patients with limited treatment options. In a phase I/II study, nivolumab alone and nivolumab plus ipilimumab showed comparable efficacy with durable responses in pretreated patients with SCLC [27]. Further, more recent studies have reported that addition of atezolizumab to chemotherapy in the first line treatment of ED-SCLC prolonged OS as well as PFS as compared to chemotherapy alone [28]. Moreover, studies evaluating novel drugs which are targeting DNA repair pathways, Bcl-2, or the hedgehog and NOTCH pathways, are increasing because of a greater understanding of the molecular alterations in SCLC [29-31]. With the recent development of safe and effective drugs, there are more drugs available for elderly SCLC patients with comorbidities, which makes PPS more important than PFS for initial treatment.
Although subsequent treatment has known to improve prognosis, patients’ performance, comorbidity, and elderly age are often obstacles that make it difficult to apply treatment. The SCS, which was developed for specific use in patients with NSCLC, has been reported in several studies as a predictor of prognosis of SCLC as well [9,11,32]. Our study also showed that the SCS had a greater correlation with OS or PPS than PS which NCCN guidelines recommend to consider for treatment decision. The SCS is an easily quantifiable scale of comorbidity associated with the prognosis of lung cancer and is one of the most reliable prognostic factors in patients with ED-SCLC, according to our results. However, SCS was not a predictive factor for subsequent treatment, since second line chemotherapy prolonged the OS regardless of the degree of SCS.
Although second line chemotherapy may be helpful in prolonging OS, the number of patients who actually received was less than half of the patients who underwent first line chemotherapy in this study. There are many reasons for the low number of patients treated, but the most important cause may be physician’s hesitation due to the fear of toxicity and the distrust of efficacy depending on the aggressive nature of the disease. To improve the rate of subsequent treatment, a more detailed risk-benefit assessment and a lot of evidence to support it are needed when deciding whether or not to apply second line chemotherapy to each patient. Second-line cytotoxic chemotherapy with reduced dose or immunotherapy such as nivolumab and pembrolizumab should be actively considered to reduce the risk of treatment. In addition, a large number of clinical trials are also needed to validate the efficacy of novel drugs for ensuring confidence in the effectiveness of subsequent treatment.
To our knowledge, this is the first study to demonstrate the importance of subsequent treatment using the concept of PPS and SCS in ED-SCLC. Nevertheless, there are some limitations of this study. First, the sample size is relatively small. Because it was a retrospective study, we could not control for the confounding factors that affect PPS or OS. Additionally, there may be a selection bias that includes only patients with relatively good prognosis as the study enrolled only those patients who underwent first line chemotherapy. Additionally, elderly patients were defined as 65 years or older in this study, which may be different from other studies because there is no consensus. However, many studies have applied the same criterion and we conducted multivariable analysis according to age subgroup. In addition, geriatric assessment and quality of life assessment were not possible since it was a retrospective study. Future studies should be conducted in a prospective, large-scale basis on the use of novel drugs to evaluate not only simple efficacy measurements such as OS or PPS, but also geriatric assessment or quality of life assessment.
In conclusion, our results showed that PPS had a higher correlation with OS than PFS in elderly patients with ED-SCLC. The most important prognostic factors for PPS and OS included SCS and second line chemotherapy. Patients receiving subsequent treatment had increased OS regardless of the degree of SCS. Therefore, we need to actively consider subsequent chemotherapy even in elderly patients with ED-SCLC irrespective of comorbidity.
KEY MESSAGE
1. Subsequent chemotherapy may be important to improve the overall survival of extensive stage small cell lung cancer even in elderly patients with underlying comorbidities.
2. Post-progression survival might be more predictive in extensive stage small cell lung cancer in elderly.
Notes
No potential conflict of interest relevant to this article was reported.