Role of concurrent chemoradiation on locally advanced unresectable adenoid cystic carcinoma
Article information
Abstract
Background/Aims
Adenoid cystic carcinoma (ACC) is a rare salivary gland tumor characterized by indolence, with a high rate of local recurrence and distant metastasis. This study aimed to investigate the effect of concurrent chemoradiation (CCRT) on locally advanced unresectable ACC.
Methods
We retrospectively analyzed clinical data from 10 patients with pathologically confirmed ACC of the head and neck who received CCRT with cisplatin in Seoul National University Hospital between 2013 and 2018.
Results
Ten patients with unresectable disease at the time of diagnosis or with positive margins after surgical resection received CCRT with weekly cisplatin. Eight patients (80%) achieved complete remission, of which three later developed distant metastases without local relapse; one patient developed distant metastasis and local relapse. Two patient achieved partial remission without progression. Patients experienced several toxicities, including dry mouth, radiation dermatitis, nausea, and salivary gland inflammation of mostly grade 1 to 2. Only one patient showed grade 3 oral mucositis. Median relapse-free survival was 34.5 months (95% confidence interval, 22.8 months to not reached).
Conclusions
CCRT with cisplatin is effective for local control of ACC with manageable toxicity and may be an effective treatment option for locally advanced unresectable ACC.
INTRODUCTION
Adenoid cystic carcinoma (ACC) is a rare malignant tumor that mainly occurs in salivary glands but can develop in various tissues of the body including the trachea, sinus, breast, and lung [1-3]. ACC constitutes 10% of salivary gland tumors and approximately 1% of head and neck tumors [4]. ACC progresses slowly but has a high rate of perineural invasion, local recurrence, and indolent distant metastasis [5,6].
The treatment of choice for ACC without distant metastasis is complete surgical excision with negative resection margin [7]. However, in locally advanced cases, ACC frequently invades the surrounding anatomy of the head and neck, and wide excision with sufficient margin is often difficult [6]. Patients with ACC respond well to radiotherapy, and radical radiation therapy (RT) has been applied in unresectable cases [8]. It is not clear whether radical RT alone improves survival outcomes or relieve symptoms in locally advanced ACC. Several cases have been reported with promising results from concurrent chemoradiation (CCRT) [9,10].
Cisplatin is well known for its role as a radiosensitizer for several malignancies, owing to its interference with DNA double-strand break repair [11,12]. Theoretically, radical CCRT may be more effective than radical RT alone, for local disease control in ACC. However, the effect of CCRT on ACC has not been fully established.
This study aimed to evaluate the efficacy and feasibility of CCRT in locally advanced unresectable ACC.
METHODS
Patient population
Medical records were reviewed retrospectively for patients who were diagnosed with locally advanced unresectable ACC and treated with CCRT at Seoul National University Hospital between 2013 and 2018. Diagnosis was confirmed pathologically. TNM stage of patients was established according to the 8th edition of the American Joint Committee on Cancer (AJCC). In brief, the AJCC 8th edition defines stage T3 as a tumor invading soft tissue and stage T4 as a tumor invading a nearby structure, such as the jaw bone, skull, skin, or nerves. Each case was presented for multidisciplinary evaluation before making recommendations for definitive therapy. Unresectable status was determined by the multidisciplinary team, comprising head and neck cancer experts from Seoul National University Hospital.
Adult patients aged 18 years or older with more than one measurable lesion according to the Response Evaluation Criteria for Solid Tumors (RECIST 1.1) [13], an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 to 2, and adequate hematologic, hepatic, and renal function were included for analysis.
Treatment and analysis
CCRT consisted of conventional fractionated radiotherapy for the primary tumor ± regional lymph nodes, with concurrent intravenous cisplatin chemotherapy. Nine patients received RT using volumetric-modulated arc therapy. Only one patient received intensity-modulated radiotherapy with simultaneous integrated boost. Four patients received 21 to 33 fractionated doses of RT to only the primary tumor site. Six patients received 30 fractionated doses of RT to both the primary site and around the tumor structure. Details about dose and fractions are summarized in Table 1. Nine patients received 35 mg/m2 of intravenous cisplatin for 6 cycles every week, and one patient received 100 mg/m2 of cisplatin for 3 cycles every 3 weeks. Responses were evaluated according to RECIST 1.1, and toxicity was assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v5.0).
Relapse-free survival (RFS) was defined as the time from the first day of CCRT to the date of relapse, disease progression, distant metastasis, or death. Median RFS, overall survival (OS), and median follow-up period were calculated using the Kaplan-Meier method.
Ethics
The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (No.: H-1812-059-993). All studies were conducted according to guidelines for biomedical research and Declaration of Helsinki. The requirement for informed consent from patients was waived because this study was retrospective.
RESULTS
Patient characteristics
Three patients were male and seven were female. Median age at the time of diagnosis was 55 years (range, 46.8 to 60.6). Seven patients were ineligible for resection because of bulky mass, perineural invasion, and inoperable location. Two patients declined surgery due to surgical morbidity. The remaining patient received surgical resection with positive margins, and the tumor was subsequently determined to be unresectable by the multidisciplinary team because further resection was impossible. Table 1 lists the reasons for unresectable status.
The primary tumor site was the parotid gland in three patients, the palate in two patients, and the sublingual salivary gland, buccal, maxillary sinus, external auditory tract, and trachea in the remaining patients. Only one patient had positive node involvement, and none showed distant metastasis. The median follow-up period was 35.7 months (range, 8.3 to 68.8).
Treatment outcomes
Patients received a median of 69.8 Gy (range, 44.5 to 72.0) of radiation. Four patients received radiation at the primary tumor site, and six patients received radiation at both the primary site and the surrounding structure. Eight patients achieved complete remission (CR), and two patients achieved partial remission (PR) (Fig. 1). Local recurrence was observed in one patient who received the lowest dose of radiation. Four patients developed lung metastasis after CCRT (Table 1). One patient received radiofrequency ablation, and two patients underwent video-assisted thoracoscopic surgery (VATS) for single lung metastasis. One patient with multiple lung metastases underwent systemic therapy with axitinib. Among four patients with metastasis, two showed progression of lung lesion without local progression of the primary tumor, and the two patients who underwent VATS retained no evidence of disease. All patients were alive. Median RFS was 34.5 months (95% confidence interval, 21.8 to not reached) (Fig. 2). Median OS was not reached (95% confidence interval, not reached to not reached).

Representative image before and after concurrent chemoradiation. (A) Patient No. 1: light parotid gland mass disappeared after concurrent chemoradiotherapy (CCRT). (B) Patient No. 6: adenoid cystic carcinoma of maxillary sinus disappeared after CCRT.
Adverse events
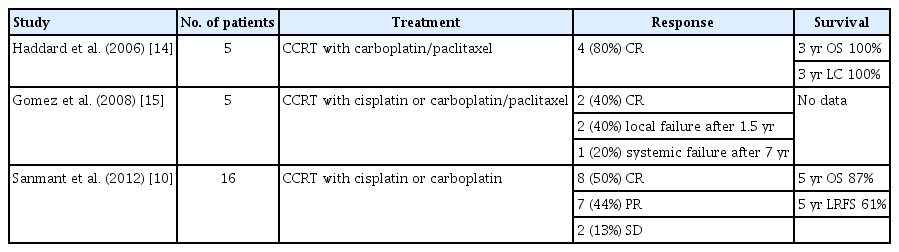
The most common toxicities of CCRT with cisplatin were nausea (n = 4) and radiation dermatitis (n = 3). Dry mouth (xerostomia), pruritis, and salivary gland inflammation were reported in two patients, respectively. Two patients showed grade 3 oral mucositis (Table 2) [10,14,15]. All toxicities were tolerable.
DISCUSSION
In this study, locally advanced unresectable ACC patients receiving radical CCRT had a high CR rate of 80% with low local recurrence.
The primary treatment for ACC is complete surgical resection, but it is often difficult to perform surgery due to the invasion of the surrounding structures such as the nerve, skull base, and brain [16,17]. ACC of the trachea, nasopharynx, or maxillary sinus that are unable to undergo surgery have been treated with radiotherapy [18]. The effect of definitive RT for unresectable cases has been reported from several retrospective studies, with a response rate of approximately 40% and RFS of approximately 30 months [19-21]. However, it is difficult to obtain long-term remission in unresectable cases by RT alone [22]. There have been a small number of case reports regarding the effects of CCRT, indicating a tolerable local control rate for ACC [10,23-25]. Ten patients diagnosed with ACC received CCRT in our study. Among them, eight patients (80%) achieved CR, experiencing long-term locoregional control with no local progression or relapse, and the remaining patients showed PR. Our results are in line with those of previous reports [23-25], suggesting that CCRT could be more effective than radical RT alone. CCRT with cisplatin may be a good treatment option for patients with unresectable ACC.
ACC of the salivary gland is associated with an increased risk of a positive margin following resection, compared to adenocarcinoma, and is therefore associated with poor prognosis [26]. Although one study reported that surgery alone provided long-term survival rates comparable to those of surgery plus radiotherapy, others found that radiotherapy was effective in cases involving positive margins [27-29]. In our study, a patient receiving postoperative CCRT achieved CR, followed by systemic relapse but not local recurrence. CCRT can therefore be expected to affect local disease control following surgery.
Cisplatin is used as a radiosensitizer in CCRT for many cancers. However, CCRT with cisplatin often fails to proceed as planned due to toxicity [30,31]. Recently, platinum-based CCRT has been reported to be effective for nasopharyngeal cancer [32,33]. All patients in this study received cisplatin with radiotherapy. Nine patients were injected with cisplatin dose of 35 mg/m2 in 6 cycles per week. One patient received high-dose cisplatin every 3 weeks. Patients experienced toxicities including dermatitis, xerostomia, nausea, and changes in taste, but most toxicities were tolerable at grade 1 or 2. CCRT with cisplatin is therefore effective with tolerable toxicity.
Our study has several limitations. This study was retrospective and analyzed a small number of just 10 cases; all cases were from a single center, and CCRT was not compared with radical radiotherapy. However, considering the rare incidence of ACC, a small number of studies will still be meaningful. No previous studies have analyzed the effect of CCRT with cisplatin in the same clinical setting, so our study will help select CCRT as a treatment option.
In conclusion, this study suggests that CCRT with cisplatin may be an effective treatment option with manageable toxicity for locally advanced unresectable ACC. Large-cohort prospective studies are needed to confirm efficacy, and a randomized trial to compare CCRT with radical RT alone is warranted.
KEY MESSAGE
1. Concurrent chemoradiotherapy (CCRT) with cisplatin provides a good local control rate with low recurrence in locally advanced unresectable adenoid cystic carcinoma (ACC).
2. CCRT with cisplatin has manageable toxicity in locally advanced unresectable ACC.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We thank the patients included in the current study. This study was supported by a grant from the Korea Health Technology R&D Project “Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer” through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare (MHW), Republic of Korea (grant number HI17C2085).