Environmental risk factors and comorbidities of primary biliary cholangitis in Korea: a case-control study
Article information
Abstract
Background/Aims
The risk factors for the development of primary biliary cholangitis (PBC) is unclear. This study aimed to investigate the risk factors associated with PBC in Korea through a questionnaire survey.
Methods
Consecutively enrolled 103 PBC patients from six referral hospitals and 100 age- and sex-matched community controls participated in this study. A standardized questionnaire survey including demographics, lifestyle, individual and familial medical history and reproductive history was prospectively collected and analyzed.
Results
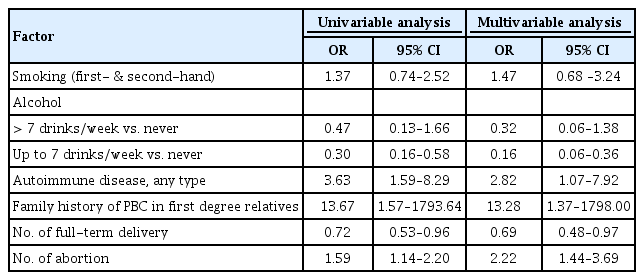
The PBC patients had a mean age of 58.3 years and a female proportion of 86.4%. The age- and sex-matched controls had a similar educational level and economic status to the PBC patients. Among the lifestyle factors, the multivariable analysis showed smoking including both first-hand and second-hand (odds ratio [OR], 2.03; 95% confidence interval [CI], 1.06 to 3.93), history of autoimmune diseases (OR, 2.46; 95% CI, 1.06 to 6.35), and family history of PBC (OR, 17.76; 95% CI, 1.77 to 2,418.74) were significantly associated with PBC, whereas alcohol intake was negatively associated with PBC. Among reproductive factors, the number of induced abortions was significantly associated with PBC, while the number of full-term deliveries was negatively associated with PBC.
Conclusions
A family history of PBC, accompanying autoimmune diseases, and smoking were significantly associated with PBC. More induced abortions and less full-term deliveries were associated with PBC in women. In contrast, mild to moderate alcohol intake was negatively associated with PBC. Further studies are warranted to validate the results of this study and to search for clues about the pathogenesis of PBC.
INTRODUCTION
Primary biliary cholangitis (PBC) is a rare, chronic inflammatory autoimmune disease that mainly affects middle-aged women [1]. It is characterized by non-suppurative inflammation and destruction of the interlobular bile ducts leading to cholestasis and cirrhosis and the presence of the anti-mitochondrial antibody (AMA) in serum [1].
The prevalence of PBC varies widely depending on the geographic location, ranging from 1.91 to 40.2 per 100,000 inhabitants [2], which suggests certain genetic and environmental factors influence the pathogenesis of PBC. Though the pathogenesis of PBC still remains enigmatic, an organ-specific autoimmune reaction triggered by exposure to putative environmental factors in genetically susceptible individuals is a convincing explanation [3].
Previous studies regarding the environmental risk factors of PBC have been conducted mostly in Western countries with a relatively high prevalence of PBC. Those studies reported that smoking [4-7] and recurrent urinary tract infection (UTI) were frequently associated with PBC. However, the extent of exposure to environmental factors such as smoking, alcohol drinking, use of cosmetics, or contraceptive measures is variable depending on the countries and regions with different epidemiologies and life styles. In this case-control study, we investigated the environmental risk factors and comorbidities associated with PBC in South Korea.
METHODS
Study population
One hundred-three patients with PBC from six referral centers in South Korea were prospectively enrolled from August 2014 to March 2015. The diagnostic criteria for PBC were at least two of the following findings present in the patients; chronic cholestasis, presence of AMA and liver histology compatible with PBC. The Institutional Review Board (IRB) approved this study (Ilsan Paik Hospital IRB number 2014-08-233) and written informed consent was received from all participants. The patients diagnosed as autoimmune hepatitis-PBC overlap syndrome were excluded.
As a control group, 100 individuals matched to the PBC cases according to a stratified sampling based on age (10-year age groups) and gender were recruited from the commercialized Macromill Embrain research panel (Macromill Embrain, Seoul, Korea; www.embrain.com), which consists of 1,088,408 individuals representative of the Korean national population. The participants were enrolled on a voluntary basis. The survey was conducted anonymously between August 25 and September 26, 2014.
Questionnaire survey
A standardized questionnaire survey was administered that included 137 questions regarding demographic, anthropometric, life style, medical and familial factors, reproductive (only for female) and socioeconomic factors. Demographics included education and subjective socioeconomic class. Lifestyle factors included first- and second-hand smoke, alcohol consumption (drinks per day, frequency and duration), coffee consumption, and use of cosmetics and hair dye. Medical history included autoimmune diseases such as autoimmune hepatitis, thyroid illness, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, Sjogren’s disease, and inflammatory bowel diseases, infectious disease such as tuberculosis, clonorchiasis and measles, operations and vaccinations. Reproductive factors included age of menarche and menopause, oral contraceptives, hormone replacement therapy, induced abortion, miscarriage, full-term delivery, and breast feeding. The details of the questionnaire (in Korean) is presented in the Supplementary Material.
PBC patients were interviewed face-to-face by a research coordinator or attending physician at each of the centers. Controls were interviewed face-to-face by trained persons hired by Macromill Embrain.
Statistical analysis
Continuous variables were expressed as the median (interquartile range) or mean ± SD as appropriate. Categorical variables are presented in absolute and percentage values (%). Continuous variables were compared using the Student’s t test, or Mann-Whitney U test as appropriate, and categorical variables were compared using the chi-square test or Fisher’s exact test. Multivariable logistic regression analyses with the Firth method for rare events were performed for variables with a p < 0.1 in the univariable analysis and for clinically relevant variables with p > 0.1, but which were consistently reported as significant variables in other studies (i.e., smoking and UTI) to identify the risk factors of PBC for all subjects and a female subgroup, respectively. A p < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 25 (IBM Co., Armonk, NY, USA).
RESULTS
Comparison of the demographic, socioeconomic, lifestyle factors, and medical history between the PBC patients and control group
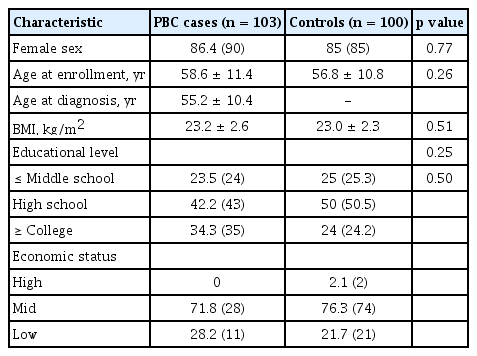
The mean age of the case and control group was 58.6 ± 11.4 and 56.8 ± 10.8 years, respectively. The female proportion of the case and control group was 86.4% and 85%, respectively. Educational level and economic status were similar between the two groups (Table 1).
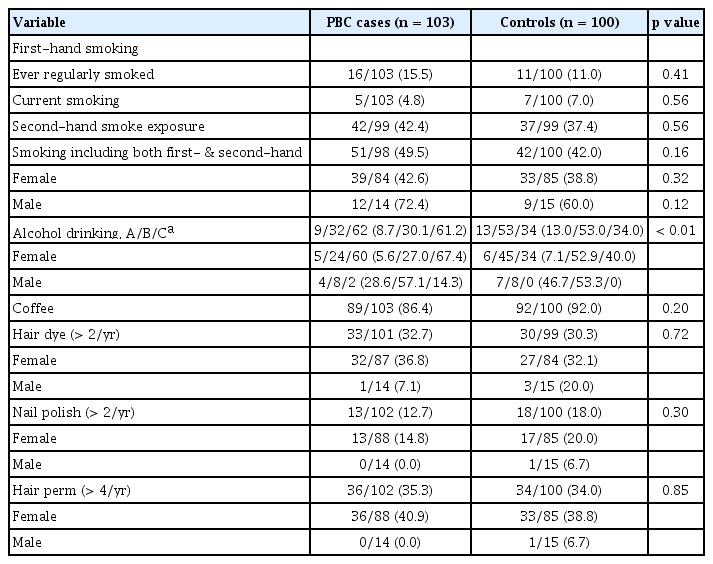
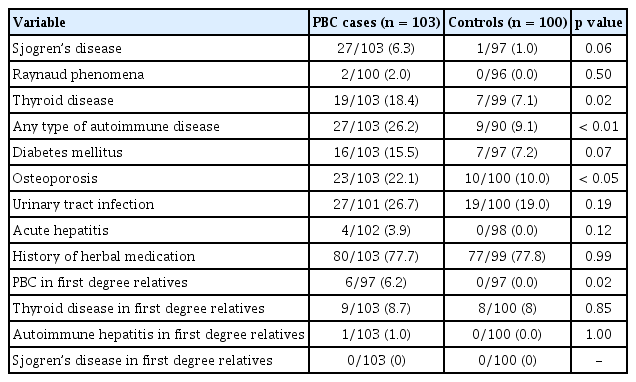
The proportions of ever-smokers including current and ex-smoker (15.5% vs. 11.0%) were low in both groups and not statistically different between the PBC and controls groups (Table 2). Although the proportions of those who have experienced first-hand and/or second-hand smoking tended to be higher in the PBC patients (49.5%) than in the control (42.0%) group, it was not significantly different (p = 0.16). The proportions of ever-drinkers and persons who have had more than seven drinks per week were significantly lower in the PBC groups than in the controls (p < 0.01). Coffee drinking and exposure to nail polish, cosmetics and hair dye were not significantly different between the two groups. The prevalence of PBC in first degree relatives was higher in the PBC cases (6.2% vs. 0%, p = 0.02). The prevalence of other autoimmune diseases in first degree relatives did not differ between the relatives of the PBCs cases and those of the control group (Table 3).
History of any autoimmune diseases including autoimmune thyroiditis, Sjogren’s disease, autoimmune hepatitis, systemic sclerosis and others was more frequent in the PBC cases than in the control group (26.2% vs. 9.1%, p < 0.01). Among the autoimmune diseases, autoimmune thyroiditis (18.5% vs. 7.1%) and Sjogren’s disease (6.3% vs. 1.0%) were significantly more prevalent in the PBC cases. Diabetes seemed to be more frequent in the PBC cases than in the controls (15.5% vs. 7.2%, p = 0.07). Medication for osteoporosis was more frequent in the PBC cases than in the controls. However, history of UTI as a possible risk factor of PBC in previous studies did not differ between the two groups. History of herbal medication was similarly frequent in both groups (78.6% vs. 77.8%) (Table 3). The frequencies for a positive history of all types of malignancies, acute hepatitis, tuberculosis, clonorchiasis, appendectomy, cholecystectomy, tonsillectomy, and vaccination for hepatitis and tuberculosis were not different between the two groups.
Comparison of the reproductive and gynecological factors between the PBC patients and the control group among women
Age at menarche and menopause was similar between both groups. The PBC patients (74.2%) had a similar frequency in menopause state as the control group (62.5%). Experience with both pregnancy (91% vs. 100%, p < 0.05) and full-term delivery (91% vs. 100%, p < 0.05) was less frequent in the PBC cases than in the controls. Moreover, the total number of full-term deliveries was lower in the PBC cases than in the controls (2.1 ± 1.2 vs. 2.5 ± 0.9, p < 0.05). Experience of receiving an induced abortion tended to be higher in the PBC groups than in the controls (51.1% vs. 37.7%, p = 0.07), and number of abortions was significantly higher in the PBC groups (1.2 ± 2.1 vs. 0.5 ± 0.7, p < 0.05). However, spontaneous abortions were not significantly different between the two groups. The frequencies of hormonal replacement therapy for postmenopausal syndrome and past use of oral contraceptives were similar between the two groups (Table 4). Pruritus and abnormal liver biochemistry during pregnancy were not different between the two groups, either.
Multivariable analysis of the risk factors for PBC
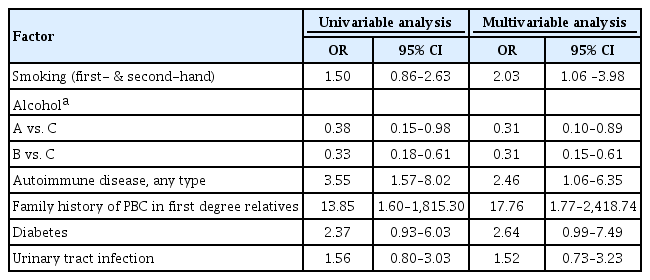
Results of the univariable and multivariable analyses among the whole subjects are shown in Table 5. In multivariable analysis, family history of PBC, history of other autoimmune diseases, and smoking including first- and second-hand were significantly associated with PBC, but alcohol drinking was negatively associated with PBC.
As a subgroup analysis including only women, the multivariable analysis showed that the presence of family history of PBC, history of other autoimmune diseases, and induced abortions were significantly associated with PBC, but alcohol dinking less than seven drinks per week and number of full-term deliveries were negatively associated with PBC (Table 6).
DISCUSSION
In this age-and sex-matched case-control study using a face-to-face questionnaire interview, family history of PBC, history of autoimmune diseases, smoking including both first-hand and second-hand, and induced abortions in women were significant risk factors, but alcohol drinking and full-term delivery in women were protective for PBC in South Korea, where the prevalence of PBC is low as 4.75 per 100,000 inhabitants [8].
The pathogenesis of PBC is still unknown but is thought to be triggered by certain environmental factors which drive the loss of tolerance to mitochondrial antigens of small bile ducts in genetically susceptible hosts. The role of genetic factors in the pathogenesis of PBC is suggested by monozygotic twin concordance [9], a higher prevalence of PBC and other autoimmune diseases in first degree relatives [10], and genetic studies on major histocompatibility [11]. Environmental factors in the pathogenesis of PBC has been investigated mainly by case-control studies using questionnaire surveys on demographics, life style, medical history, and family history. Among these factors, smoking was consistently associated with PBC [4-7] or advanced fibrosis in PBC [12]. Most studies have shown that the history of recurrent UTI was positively associated with PBC [4-7], while alcohol intake was negatively associated with PBC [6,7]. Some studies have reported with controversy that abortions [5], exogenous estrogens [7], and frequent use of nail polish [7] or hair dye [6] were associated with an increased risk of the disease.
In our study, the first-hand smoking rate was not significantly associated with PBC in this study. However, smoking including both first-hand and second-hand was significantly associated with PBC in the multivariable analysis, although this association was abolished in the multivariable analysis only for women that included gynecological and reproductive factors. The smoking rates in our study are much lower (15.5%) than those in the previous studies (45% to 53%) [4,5] in which smoking was a significant risk factor of PBC. Moreover, the smoking rate in Korean women is much lower than in women in other countries with a high prevalence of PBC [13]. Several studies reported that cigarette smoking was not only a risk factor of PBC [4,6,7,14,15], but also a factor for advanced fibrosis in PBC in a dose-dependent manner [13], while only a few studies found no association between smoking and PBC [10,16]. Smoking is generally a profibrotic factor for advanced fibrosis in non-alcoholic fatty liver disease as well as alcoholic and viral liver disease, probably due to free radical generation, oxidative stress or hypoxia [17]. However, some discrepancies in the association of smoking and PBC might be due to the study design or sample size. Smoking is considered a contributing factor to PBC pathogenesis.
This study showed that alcohol drinking was negatively associated with PBC, which was compatible with previous studies [6,7]. The negative association between PBC and alcohol intake might result from recall bias or a reduction in alcohol intake after a patient has been diagnosed with a liver disease. However, considering that the proportion of never-drinkers was much higher in the PBC cases, alcohol drinking was not likely to merely be a confounding factor. Moreover, the protective effects of low to moderate alcohol drinking was suggested in systemic lupus erythematosus, rheumatoid arthritis and autoimmune hepatitis [18,19]. Therefore, the association of alcohol and PBC needs to be explored in further studies.
In keeping with previous studies, PBC was frequently associated with autoimmune diseases including autoimmune thyroiditis and Sjogren’s disease in this study. First degree relatives of PBC patients showed a higher rate of diagnosis of PBC than of those in the control group (6.2% vs. 0%) because familial clustering cases with four sibling PBC cases were included in this study [20]. It might lead to a stronger association between family history of PBC in this study compared with other studies in which the prevalence of PBC in first degree relatives was reported to be 1.33% to 6.40% [21].
Many studies reported that recurrent UTI was a significant risk factor of PBC [5-7,15]. Escherichia coli, a major microorganism causing UTI, can be a strong inducer of pyruvate dehydrogenase complexes (PDC)-E2 specific AMA and liver pathology consistent with PBC in a mouse model [22]. However, ever or multiple UTIs were not associated with PBC in this study. Notably, the UTI rates in this study (26.7%) were much lower than in other studies (39% to 70%) [4,15]. It was not clear whether the lower rate of UTIs reflected a really low incidence in Koreans or resulted from a recall bias; therefore, further validation is required.
Not only bacterial infection, but also xenobiotics may break immune tolerance by the mechanism of molecular mimicry. Octynoic acid, commonly used in artificial flavoring and cosmetics, was shown to induce AMA and PBC-like disease in murine models [23]. Moreover, certain xenobiotics such as nail polish [7] and hair dye [6] were reported to be associated with PBC. However, there were no clear associations between the use of xenobiotics including cosmetics, hair dye, perm agent, nail polish, and herbal drugs in this study.
Because PBC is a female predominant disease, many studies have explored the association between hormonal or reproductive factors and PBC. Some study reported gravidity [24], exogenous estrogen [7], and pruritus during pregnancy [5,6] were associated with PBC, and other reported oral contraceptives [5] were protective against PBC development. Most results on reproductive factors have not been reproduced in other studies. In this study, the proportion of females who had experienced receiving an induced abortion was strikingly high, and it was significantly more frequent in PBC patients than in the controls. The rate of induced abortions in Korea was reported to be up to 50% in 1980s and 1990s [25], whereas the use of oral contraceptives was reported to be 6% in 1984 [26] although it increased to 31.3% in 2007 in Korea [27]. Corpechot et al. [5] also reported that abortion was a risk factor of PBC. Moreover, induced abortion was reported to be a risk factor in autoimmune thyroiditis [28]. Fetal microchimerism, which occurs more frequently in surgical abortions than in spontaneous abortions, was assumed to be associated with the development of autoimmune diseases [29]. Therefore, the association between PBC and abortion should be studied further. Cultural differences in contraception methods might lead to the different association between PBC and reproductive factors among countries. Unexpectedly, the number as well as the experience of a full-term delivery was negatively associated with PBC. There have been few studies on the association between PBC and full-term delivery or gravity. One study showed that PBC patients reported significantly more pregnancies which is contradictory to our results. This association also needs to be identified further.
The limitations of this study include an interview-based questionnaire survey which has inherently a recall bias and a relatively small sample size. In addition, the PBC patients were enrolled from tertiary referral centers and might not be representative of PBC patients in general. Lastly, comorbidities and past illness can be underestimated because the PBC patients were interviewed by medical personnel, while the controls were interviewed by non-medical professional interviewers working for a commercial survey company, although we used the same questionnaires with detailed explanations and education to minimize the heterogeneity among the interviewers. Despite these limitations, this is the first study to our knowledge on the risk factors of PBC in Asian countries. Moreover, this is a multicenter study using face-to-face interviews for both the patients and the controls with comprehensive and detailed questionnaires that included reported risk factors on PBC.
In summary, we found that smoking, history of autoimmune disease, and artificial abortions were risk factors of PBC, while alcohol drinking and full-term delivery were negatively associated with PBC in South Korea. Further studies to validate the results of this study and search for clues on the pathogenesis of PBC are warranted.
KEY MESSAGE
1. A family history of primary biliary cholangitis (PBC), accompanying autoimmune diseases, and smoking were signif icantly associated with PBC in this case-control study.
2. More induced abortions and less full-term deliveries were significantly associated with female PBC.
3. Mild to moderate alcohol intake was negatively associated with PBC.
Notes
No potential conflict of interest relevant to this article was reported.