Performance of pneumococcal urinary antigen test in patients with community-onset pneumonia: a propensity score-matching study
Article information
Abstract
Background/Aims
Although pneumococcal urinary antigen tests (PUATs) have universally been used for the diagnosis of pneumococcal pneumonia, data on the efficacy of these exams are limited. The objective of our study was to investigate the clinical impact of the PUAT in patients with community-onset pneumonia (CO-pneumonia).
Methods
We conducted a retrospective cohort study of patients diagnosed with CO-pneumonia. Patients were classified according to their PUAT results and were matched using the propensity score-matching method. The primary outcome was 30-day mortality.
Results
A total of 1,257 patients were identified and 163 (13.0%) demonstrated positive PUAT results. The sensitivity and specificity values of PUAT for overall pneumococcal pneumonia were 56.5% and 91.4%, respectively. In the full cohort, there were no significant differences in 30-day mortality between the two groups (6.1% in the positive PUAT group vs. 8.2% in the negative PUAT group, p = 0.357). However, in the propensity-matched cohort, the 30-day mortality rates were lower in the positive PUAT group (5.6% vs. 17.4%, p = 0.001). With respect to secondary outcomes, the proportion of patients with potentially drug-resistant pathogens, changes in antibiotics, and failure rates of initial antibiotic therapy were significantly lower in the positive PUAT group than in the negative PUAT group of the propensity-matched cohort.
Conclusions
We found that the sensitivity of the index test was low and specificity was high in this clinical setting. And our findings suggest that positive PUAT results may be associated with favorable clinical outcomes in patients with CO-pneumonia.
INTRODUCTION
Pneumonia is the leading cause of infectious disease-related deaths in adults worldwide [1]. Selecting appropriate pathogen-directed antibiotics in patients with community-onset pneumonia (CO-pneumonia) can decrease treatment cost, drug-related adverse events, and antibiotic resistance and allow for the narrowing of empiric antibiotic therapy [2-4]. Despite the development of diagnostic techniques for pneumonia, causative organisms have not been detected in more than half of the patients [5]. Determining the etiology of pneumonia has remained challenging because of several reasons, including difficulties in obtaining good-quality sputum and the unreliability of subsequent culture results, low sensitivity of blood cultures, and the administration of antibiotics before sample collection [5,6]. Streptococcus pneumoniae is the most frequently isolated pathogen; therefore, initial empirical antibiotic treatment regimens for most patients with community-acquired pneumonia (CAP) aim at covering S. pneumoniae [3]. The early identification of S. pneumoniae may lead to the selection of more reliable pathogen-targeted antibiotics [3].
The pneumococcal urinary antigen test (PUAT) is an assay widely used to identify the C-polysaccharide antigen common to all serotypes excreted into the urine using a membrane immunochromatographic test [7]. In addition to the advantages of rapidity and simplicity, it has a high sensitivity and specificity over Gram stain and sputum cultures, as well as availability in patients who cannot demonstrate expectorated sputum [3]. In addition, even after antibiotic therapy has been started, the PUAT has the ability to detect pneumococcal pneumonia [3,8]. Current guidelines recommend that the PUAT can only be performed in patients with severe CAP or moderate- or high-severity pneumonia [3,9].
To date, the clinical benefits derived from the results of PUAT have not been fully established [10]. Thus, the aim of our study was to investigate the diagnostic performances of the PUAT, and the association between the results of PUAT and clinical outcomes in patients with CO-pneumonia.
METHODS
Study design and population
We conducted a retrospective cohort study of patients with CO-pneumonia at a university affiliated medical center. Adult patients (aged ≥ 18 years) who were hospitalized with pneumonia between January 2012 and December 2015 were investigated. Patients were identified by the use of the international diagnostic codes version 10 to screen for possible cases as follows: J18.0 to 18.9 as representative codes of pneumonia in the primary discharge diagnosis [11]. After reviewing the medical records and radiological findings of relevant patients, we confirmed the diagnosis of pneumonia as the presence of a new infiltrate on a chest radiograph and by clinical signs [2]. We excluded the following types of patients: (1) those who were readmitted due to pneumonia within 10 days of leaving the hospital; (2) those who were transferred from other hospitals after hospitalization for > 48 hours; (3) those with obstructive pneumonia; (4) those who had immunocompromised status, such as neutropenia (absolute neutrophil count < 1,500 cells/μL) after chemotherapy or human immunodeficiency virus infection; (5) those who did not receive guideline-concordant antibiotic therapy; and (6) those who had hospital- acquired pneumonia (HAP). Although the concept of healthcare-associated pneumonia (HCAP) was eliminated in the revised 2016 American Thoracic Society (ATS)/ Infectious Disease Society of America (IDSA) guidelines for the management of HAP and ventilator-acquired pneumonia [12], we included HCAP as a category of CO-pneumonia in the present study.
Urine samples were collected in the emergency department or ward in the first 24 hours to detect urinary antigens for S. pneumoniae using BinaxNOW (Binax Inc., Scarborough, ME, USA). According to the results of PUAT, we classified the study patients into positive and negative PUAT groups. Empiricalguideline-concordant antibiotics were maintained without regard to the results of tests. Demographics, radiological findings, laboratory findings, microbiological results, and clinical outcomes were compared between the two groups.
Ethical consideration
Ethical committees approved to review clinical data of relevant patients obtained from medical records such as clinical parameters, laboratory, radiological, and microbiological findings. Information obtained during the study was kept confidential and only intended for research purpose.
The study protocol was approved by the Ethical Review Committee of Jeju National University Hospital (Institutional Review Board no. 2018-04-001). Informed consent was waived because of the retrospective nature of the study.
Microbiology and antibiotics
Sampling to determine the microbial etiology of pneumonia included sputum, tracheobronchial aspirates, bronchoalveolar lavage fluid, pleural fluid, or blood through a semi-quantitative manner. The antibiotic sensitivity of all isolates was determined using a disc diffusion method. Serologic tests were performed to detect antibodies against Mycoplasma pneumoniae or Chlamydia pneumoniae. A patient was considered to have a potentially drug-resistant (PDR) pathogen if one of the following pathogens was isolated: methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, extended-spectrum beta-lactamase (ESBL)-producing or carbapenem-resistant Klebsiella pneumoniae and Escherichia coli, Acinetobacter baumannii, or Stenotrophomonas maltophilia [13].
Changes in antibiotic regimens were defined as either escalation or de-escalation after culture sensitivities or clinical stabilities were identified. Inappropriate antibiotic therapy was noted if the empirical antibiotic treatment was not effective against the identified pathogen based on in vitro susceptibility testing [14]. Initial treatment failure was defined as death during initial treatment or a change of initial therapeutic agent after 48 hours due to clinical instability [15]. Guideline-concordant antibiotic therapy for CAP was defined as antibiotic regimens recommended by the 2007 ATS/IDSA guidelines for CAP: specifically, beta-lactam plus macrolide or fluoroquinolone [3]. Since the data of the present study were collected before the 2016 revised ATS/IDSA guidelines were released, guideline-concordant antibiotic therapy for HCAP was defined as antibiotic regimens recommended by the 2005 ATS/IDSA guidelines, i.e., anti-pseudomonal beta-lactam plus either a fluoroquinolone or an aminoglycoside, along with anti-MRSA coverage if risk factors were present [16].
Statistical analyses
Data are presented as median (interquartile range) for continuous variables and as a number (%) for categorical variables. Continuous variables were compared using the Student’s t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were compared using the Pearson chi-square test, and Fisher’s exact test was used when any cell contained less than five items. To evaluate the reliability of the PUAT, we calculated its sensitivity and positive and negative predictive values, using the following three different reference groups of patients as the gold standard: (1) definitive pneumococcal pneumonias (CO-pneumonia with S. pneumoniae isolated in blood or pleural fluid culture); (2) probable pneumococcal pneumonias (CO-pneumonia with S. pneumoniae as the predominant morphotype on Gram stain or culture of sputum, transtracheal aspirates, and bronchoalveolar lavage); and (3) all pneumococcal pneumonias (definite plus probable) [17]. When determining specificity, we considered the control group as all patients without a diagnosis of pneumococcal pneumonia, including those with unknown etiology. We also calculated positive and negative likelihood ratios (LRs) as a measure of the extent to which the pretest odds were altered by the test results; low negative LR (< 0.1) and high positive LR (> 10) findings were considered to be useful for ruling out and ruling in decisions, respectively [18].
To improve the balance of baseline characteristics and reduce the effects of selection bias and potential confounding in this retrospective cohort study, estimated propensity scores were used to match the positive PUAT group to the negative PUAT group. The propensity score was calculated by logistic regression analysis using the covariates of baseline characteristics (Table 1). Standardized differences were estimated for all baseline covariates before and after matching to assess prematch imbalance and postmatch balance. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). All tests were two-sided, and p values of < 0.05 were considered to be statistically significant.
RESULTS
Study population
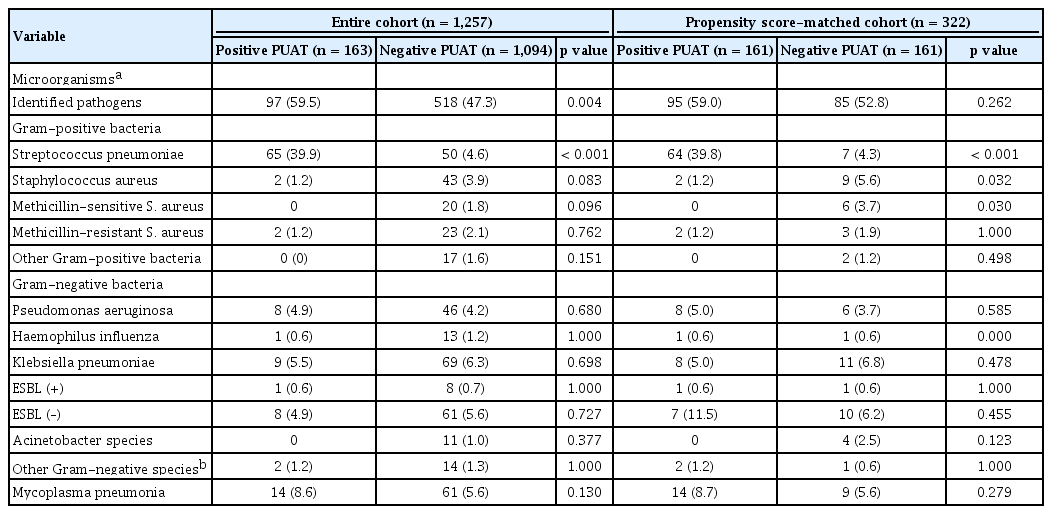
Fig. 1 shows patient enrolment. Overall, 1,257 patients were analyzed, of whom 163 (13.0%) had positive PUAT results and 1,094 (87.0%) had negative PUAT results. The proportion of patients with positive PUAT results was similar in both CAP and HCAP groups (13.1% vs. 12.8%, p = 0.875). The microbiological methods by which S. pneumoniae was identified in 115 patients as a cause of CO-pneumonia are as follows: blood cultures in seven patients (6.1%), pleural fluid in two patients (1.7%), transtracheal aspiration in 15 patients (13.0%), bronchoalveolar lavage in six patients (5.2%), and sputum in 87 patients (75.7%). In two patients, S. pneumoniae was simultaneously identified in blood and sputum cultures, and PUAT was positive in seven of nine (77.8%) patients with definitive pneumococcal pneumonia. Also, in 108 patients with probable pneumococcal pneumonia, 58 (53.7%) tested positive. Taking into account both of the groups together (i.e., all pneumococcal pneumonia), the results of PUAT was positive in 65 patients (56.5%).

Flow diagram of patient enrollment. HAP, hospital-acquired pneumonia; PUAT, pneumococcal urinary antigen test.
Baseline characteristics are presented in Table 1. Prior to matching, patients in the positive PUAT group were more likely to reside in a nursing home or long-term care facility. In addition, the median white blood cell and C-reactive protein levels were higher in the positive PUAT group than in the negative PUAT group. The propensity score-matching process provided 161 pairs of patients with positive and negative PUAT findings and achieved a good balance for all baseline comorbidities, clinical parameters, and severity indexes.
Diagnostic accuracy of the PUAT in patients with CO-pneumonia
Table 2 shows the calculated values of sensitivity, specificity, positive and negative predictive values, and positive and negative LRs by the different methods. In patients with all pneumococcal pneumonia, the sensitivity of PUAT was 56.5% (95% confidence interval [CI], 47.0 to 65.7) and the specificity was 90.9% (95% CI, 89.0 to 92.6). Additionally, the positive predictive value was 39.9% (95% CI, 34.1 to 46.0) and the negative predictive value was 95.1% (95% CI, 94.1 to 96.0). Positive and negative LRs were 6.2 (95% CI,4.9 to 8.0) and 0.5 (95% CI, 0.4 to 0.6).
Microorganisms
Table 3 shows the distributions of microorganisms on the basis of the culture results. Overall etiology was established in 97 (59.5%) and 518 (47.3%) patients in the positive PUAT and the negative PUAT groups, respectively. Among patients with positive PUAT results, other microorganisms were identified in 36 (22.1%) patients using conventional methods. In both the full and propensity- matched cohorts, the rate of S. pneumoniae identified was significantly higher in the positive PUAT group (39.9% vs. 4.6%, p < 0.001 and 39.5% vs. 4.3%, p <0.001). Following propensity score matching, the rate of Staphylococcus aureus was statistically higher in the negative PUAT group (1.2% vs. 5.6%, p = 0.032).
Associations between the PUAT and clinical outcomes
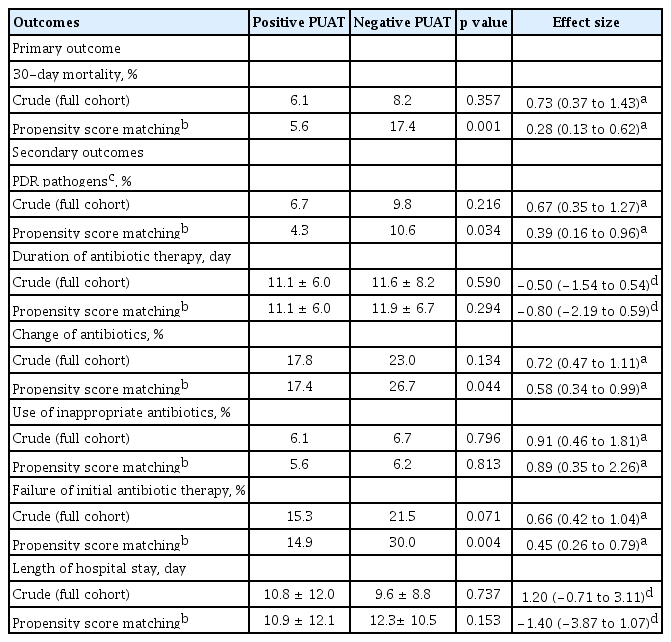
The primary and secondary study outcomes are summarized in Table 4. In the full cohort of 1,257 patients, there were no significant differences in 30-day mortality between groups (6.1% in the positive PUAT group vs. 8.2% in the negative PUAT group; odds ratio [OR], 0.73; 95% CI, 0.37 to 1.43; p = 0.357) (Fig. 2A). However, in the propensity-matched cohort, the 30-day mortality rates were lower in the positive PUAT group when compared with the negative PUAT group (5.6% vs. 17.4%; OR, 0.28; 95% CI, 0.13 to 0.62; p = 0.001) (Fig. 2B). In subgroup analysis for overall patients with severe pneumonia, the 30-day mortality was not significantly different between the positive and negative PUAT groups (23.1% vs. 33.1%, p = 0.297). On the other hand, in the propensity-matched cohort of severe pneumonia, the 30-day mortality rates were lower in the positive PUAT group (23.1% vs. 59.3%, p = 0.008).
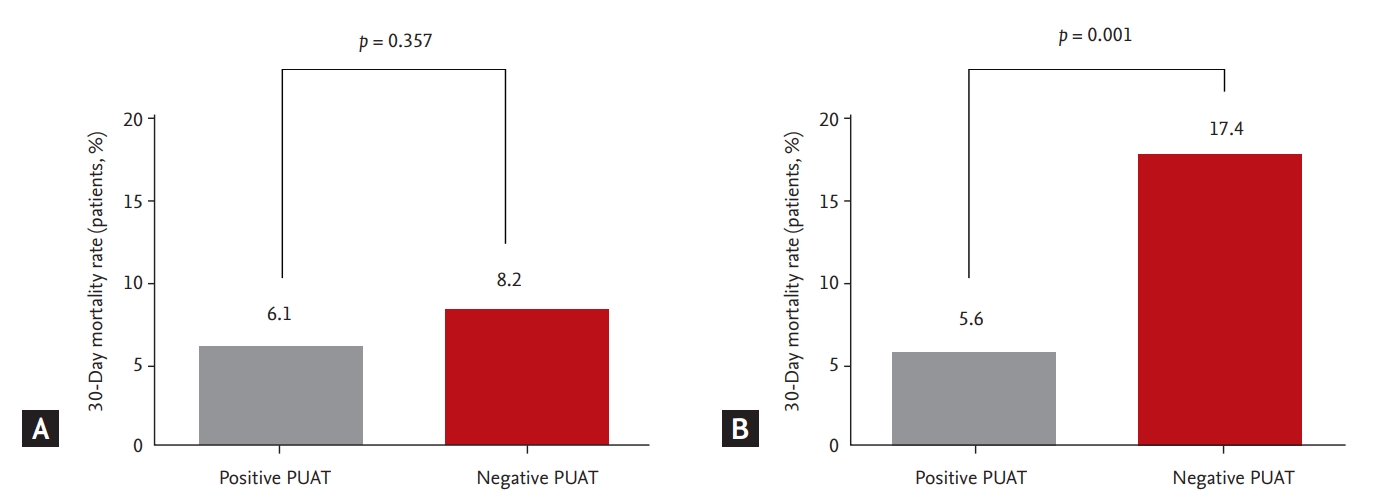
Associations between the pneumococcal urinary antigen test (PUAT) and 30-day mortality rate. (A) The full cohort and (B) the propensity score-matched cohort.
In the full cohort, there were no significant differences in all secondary study outcomes between groups. In the propensity-matched cohort, the proportion of patients with PDR pathogens was significantly lower in the positive PUAT group (4.3% vs. 10.6%; OR, 0.39; 95% CI, 0.16 to 0.96; p = 0.034). Likewise, the proportion of patients with a change of antibiotics and failure of initial antibiotic therapy was also statistically lower in the positive PUAT group compared to the negative PUAT group ([17.4% vs. 26.7%; OR, 0.58; 95% CI, 0.34 to 0.99; p = 0.044] and [14.9% vs. 30.0%; OR, 0.45; 95% CI, 0.26 to 0.79; p = 0.004], respectively).
DISCUSSION
In our study, we identified three major findings. First, approximately 13% of patients with CO-pneumonia had positive PUAT results, and the sensitivity and specificity values for the diagnosis of definitive, probable, and overall pneumococcal pneumonia were 77.8% and 87.3%, 53.7%, and 90.9%, and 56.5% and 90.9%, respectively. Second, the clinical parameters of the positive PUAT group were not worse than in the negative PUAT group. Third, in the propensity-matched cohort, the 30-day mortality rates were lower in the positive PUAT group.
In patients with CO-pneumonia, the selection of empirical antibiotic regimen is based on the prediction of the most common pathogens [3,9]. Since the widespread use of pneumococcal conjugate vaccines was initiated, recent studies have reported a decline in S. pneumoniae as a cause of CAP [2,19,20]. However, S. pneumoniae is still the most common cause at 5% to 21% among microorganisms leading to hospitalization in patients with CAP [7,21-23]. The PUAT is simple, rapid, and useful for detecting pneumococcal pneumonia when samples for culture cannot be obtained in a timely fashion or when antibiotic therapy has already commenced [3,8]. Previously, a meta-analysis demonstrated that the pooled sensitivity and specificity of the PUAT for definitive pneumococcal pneumonia in the same clinical setting used in the present study were 75% and 80%, respectively [24]. Our results indicated that the diagnostic performances of the PUAT for definitive pneumococcal pneumonia were comparable with the results of a meta-analysis [24].
The PUAT has been reported to have a higher diagnostic yield in patients with severe pneumonia [25]. Although a previous study revealed that the PUAT was more sensitive in patients with high-risk pneumonia as compared to those without (94% vs. 63%, p < 0.001) [25], the rate of severe pneumonia in our study was similar between both groups (16.0% vs. 15.1%, p = 0.773). And, while a recent multicenter USA study reported that patients with HCAP were less likely to have positive PUAT results than those with CAP [10], there were no differences in positive PUAT results between the CAP and HCAP groups in our study.
The association between PUAT result and clinical outcomes in patients with CO-pneumonia has been investigated in a few studies [26,27]. A large prospective study further demonstrated that positive PUAT results were significantly associated with 30-day mortality, intensive care unit admission, use of mechanical ventilation, treatment failure, and adverse outcomes in patients with bacteremic pneumococcal pneumonia [26]. However, this study contained the possibility of selection bias, and patients with positive PUAT results were significantly worse in clinical parameters such as respiratory rates, arterial oxygen pressure, and pH levels, and multi-lobar involvement [26]. In the present study, we found that 30-day mortality rates were significantly lower in the positive PUAT group after adjustment for initial presentations using the propensity score-matching process. Similarly, in a recent registry-based retrospective study using a modified CRB65 (confusion, respiratory rate, blood pressure, and age ≥ 65 years) score by subtracting 1 from the scoring system if the PUAT was positive, positive PUAT results were associated with lower risk of 30-day mortality [27]. We additionally investigated the association between the PUAT results and secondary outcomes. In the propensity-matched cohort, the proportion of patients with PDR pathogens, changes in antibiotics, and failure rates of initial antibiotic therapy were significantly higher in the negative PUAT group than in the positive PUAT group.
Reasons for why PUAT is negative in some patients with pneumococcal pneumonia have been proposed to include the following: low levels of C-polysaccharide antigen and sequestration of the antigen by binding to serum antibodies in immune complexes, reduced urinary excretion of the antigen, delayed times from the onset of symptoms to diagnosis, and previous antibiotic treatment [26]. We tried to find clinical parameters of false negative PUAT results. Compared to patients with true positive PUAT results, those with false negative PUAT results had chronic heart diseaseless frequently (18.5% vs. 8.0%, p = 0.049). However, because of the small sample size (n = 165) and low statistical power (p =0.049), we could not allow for this as a crucial parameter.
There remains controversy with regard to the change of antibiotics on the basis of the results of PUAT [28,29]. In a prospective study including 219 patients with non-severe CAP, the targeted use of amoxicillin based on PUAT results was able to decrease antibiotic resistance and reduce the use of unnecessary antibiotics [28]. In contrast, another randomized controlled trial reported that S. pneumoniae-targeted therapy with positive PUAT results increased the rate of clinical relapse compared with empirical therapy [29]. Possible explanations for treatment failure may include the following: first, the possibility of polymicrobial infections cannot be excluded [29]. Especially in the case of polymicrobial infections with PDR pathogens, narrowing the antibiotic treatment can lead to treatment failure. Second, a high proportion of pathogens remain unidentified in patients with CO-pneumonia. This means that it is difficult to predict the rate of polymicrobial infections. Third, pneumococcal capsular polysaccharides could react with othermicroorganisms such as Staphylococcus, Streptococcus species, and Gram-negative strains [30]. Other microorganisms identified in patients with positive PUAT results through conventional methods, might be regarded as having cross-reactivity [30]. Possible cross-reacting microorganisms could elicit treatment failure [30]. Finally, the presence of drug-resistant S. pneumoniae may also be a plausible explanation [28]. The emergence and spread of drug-resistant S. pneumoniae have been reported in recent years, and antibiotic resistance threatens the successful management of pneumococcal pneumonia [31]. In a meta-analysis encompassing 10 studies that involved 3,430 patients, the combined relative risks of all-cause mortality for the penicillin-resistant S. pneumoniae groups was 1.29 (95% CI, 1.01 to 1.66) when compared with the penicillin-susceptible S. pneumoniae group [32].
In principle, patients with positive PUAT results would have a good response to empirical guideline-concordant antibiotic therapy, which has been associated with a significant decrease in in-hospital mortality, length of hospital stay, and the duration of parenteral therapy [33]. We maintained guideline-concordant empirical antibiotics without regard to the results of tests and investigated the association between the PUAT results and clinical outcomes in the present study.
The strength of our study is in the propensity score-matching process, although our subjects were a retrospective cohort. Meanwhile, there are some study limitations. First, because our study was conducted retrospectively involving patients admitted to a single institution, our data should be interpreted with caution. In particular, even though pneumococcal vaccination might produce a false positive result in the PUAT in clinical practice [34], we could not identify the rate of pneumococcal vaccination due to the retrospective nature of the study. Second, we did not perform viral testing in most of the included patients. Mixed infections consisted of approximately 5% to 10% of the etiology for CAP in previous studies [20,27]. Most polymicrobial agents in these studies were viral pathogens, which do not usually require specific antimicrobial therapy [20,35]. Because we did not perform routine viral testing, the role of viral agents in CO-pneumonia might be underestimated in clinical outcomes. Third, previous studies have reported the relationship between serotype of S. pneumoniae and disease outcome [36]. A pooled estimates for invasive pneumococcal disease demonstrated that serotypes 1, 7F, and 8 were associated with decreased risk ratios, while serotypes 3, 6A, 6B, 9N, and 19F were associated with increased risk ratios [36]. However, the serotype of S. pneumoniae was not investigated in this clinical setting.
In conclusion, our study revealed that the PUAT had low sensitivity and high specificity for overall pneumococcal pneumonia. After baseline characteristics were adjusted through the propensity score-matching process, empirical guideline-concordant antibiotic therapy in CO-pneumonia was associated with a lower rate of 30-day mortality in patients with positive PUAT results.
KEY MESSAGE
1. The pneumococcal urinary antigen test (PUAT) had low sensitivity and high specificity for overall pneumococcal pneumonia.
2. Baseline clinical parameters were similar between the positive PUAT and negative PUAT groups.
3. In the propensity-matched cohort, the 30-day mortality rates were lower in the positive PUAT group.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by a research grant from Jeju National University Hospital in 2018.