Clinical significance of postoperative atrial arrhythmias in patients who underwent lung transplantation
Article information
Abstract
Background/Aims
Atrial arrhythmia (AA) occasionally occurs after lung transplantation (LT); however, risk factors for AA and their impact on clinical outcomes are inconsistent. We aimed to investigate the incidence, predisposing factors, and clinical outcomes of AA after LT.
Methods
We retrospectively evaluated 153 consecutive patients who underwent LT between January 2010 and August 2016. An AA episode was defined as a documented atrial fibrillation (AF), atrial flutter, or atrial tachycardia on 12-lead electrocardiography or episodes lasting ≥ 30 seconds on telemetry monitoring.
Results
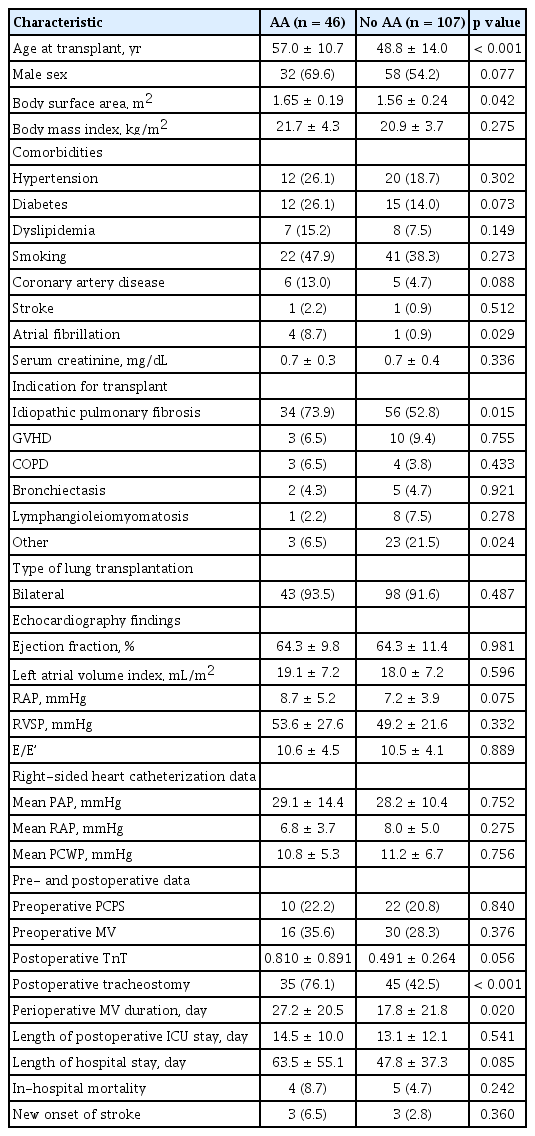
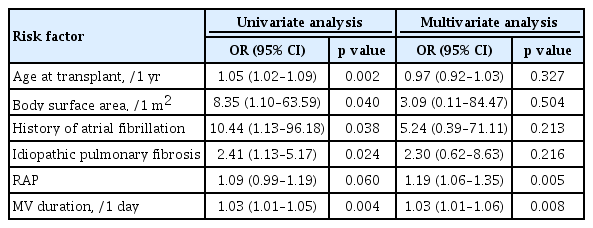
The mean follow-up time was 22.0 ± 19.1 months. Postoperative AA occurred in 46 patients (30.1%) after LT. Patients with postoperative AA were older, had larger body surface area, and had an increased incidence of paroxysmal AF prior to transplantation, idiopathic pulmonary fibrosis, and postoperative tracheostomy than patients without AA. Preoperative right atrial pressure (RAP) (odds ratio [OR], 1.19; p = 0.005) and longer periods of mechanical ventilation (OR, 1.03; p = 0.008) were found to be independent risk factors for AA after surgery. Development of AA was a significant predictor of long-term overall mortality (hazard ratio, 2.75; p = 0.017).
Conclusions
Patients with elevated preoperative RAP and long-term ventilator care had a higher risk of AA after LT. Further, AA after LT was associated with poor long-term survival.
INTRODUCTION
Lung transplantation (LT) has been performed increasingly over the past 2 decades [1,2]. Atrial arrhythmias (AA), including atrial fibrillation (AF), atrial flutter (AFL), and atrial tachycardia (AT), are highly prevalent complications after LT, with a reported incidence ranging from 25% to 45% depending on their definition and methods of detection [3-9]. At present, there is inconsistent evidence supporting the association between mortality after LT and postoperative AA [3,6,10,11]. In addition, different risk factors have been reported with inconsistent results [3,7,11]. An increasing number of LT recipients at an advanced age, bilateral nature of LT, development of donor selection criteria, donor management protocols, and postoperative management are some current noteworthy changes, especially after the implementation of the lung allocation score in 2005 [1,12], which necessitated re-evaluation of these arrhythmias. Therefore, the purpose of this study was to assess the incidence of AA in patients who underwent LT and to determine its risk factors and impact on clinical outcomes in the contemporary era.
METHODS
Patient population
This was a single-centered, retrospective cohort analysis. The study was approved by the Institutional Review Board (IRB number: 2013-0522-035) and was conducted in compliance with the Declaration of Helsinki. Informed consent and a critical event committee were exempted by the board due to this study’s retrospective design. Lung transplant patients, who underwent either single or bilateral LT, between January 2010 and August 2016, were eligible for the study. Patients who underwent heart-lung transplantation or were lost to follow-up were excluded from this study.
Data collection
Baseline characteristics, operative and postoperative clinical variables, and clinical outcomes during the follow-up period were captured from electronic medical records. Baseline demographics collected included: age, gender, comorbidities, history of AF, preoperative medications, indications for LT, electrocardiography (ECG), echocardiographic data, right heart catheterization data (if available), and laboratory results. Operation notes were reviewed to obtain details of the surgery such as transplant type (single or bilateral), surgical procedure, and intraoperative complications. Preoperative echocardiographic data included left ventricular ejection fraction (LVEF), size of the cardiac chambers, right atrial pressure (RAP), right ventricular systolic pressure, and E/E’ ratio. LVEF and left atrial volume were determined using the biplane Simpson’s method. RAP was estimated by evaluating the inferior vena cava during respiration. Right ventricular systolic pressure was calculated by adding the estimated RAP to the pressure gradient between the right atrium and right ventricle (4 × peak tricuspid regurgitant jet velocity2). Further, to determine if postoperative clinical features predicted postoperative AA, data on the duration of mechanical ventilation needed and cardiac markers were collected as well.
Study outcomes
All patients completed continuous ECG monitoring in the surgical intensive care unit (ICU). The outcomes collected included development of AA (including early and late postoperative AA), postoperative length of ICU stay, length of hospital stay, postoperative occurrences of stroke, and all-cause mortality. Early postoperative AA was defined as the first documentation of AF, AFL, or AT lasting ≥ 30 seconds via either a 12-lead ECG or rhythm strips obtained from the ECG monitoring within 30 days of transplantation. Late AA was defined as the occurrence of AA, after 30 days of transplantation, at any time during the follow-up period. ECGs were analyzed by two independent cardiologists (B.G.K. and J.S.U.). Progress notes, emergency room records, and consultation notes were also intensively reviewed to determine the physician’s assessment of arrhythmia or tachycardia. Onset time of postoperative AA was defined as the number of days from the date of LT to the date of the first episode of AA. Lengths of ICU stay and hospital stay were calculated from the date of LT to the date of transfer to the general ward and discharge, respectively. Postoperative occurrence of stroke was defined as occurrence of a focal neurological deficit confirmed from abnormal findings of brain imaging studies by a neurologist after surgery. All-cause mortality included any death after LT. Treatment strategies for AA episodes were stratified into rate control (β-blockers, non-dihydropyridine calcium channel blockers, and digoxin) and rhythm control (anti-arrhythmic drugs and electrical cardioversion). Decisions regarding treatments were at the discretion of the transplant surgeons or consulting cardiologists.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and categorical variables were presented as number and percentage. Mean differences between groups were tested using the Student’s t test for normally distributed data or the Mann-Whitney U test for skewed data. Categorical variables were analyzed using the Pearson’s chi-square test or Fisher’s exact test. Uni- and multivariate analyses using logistic regression models were constructed to validate predictors of AA. Variables with p < 0.1 from the univariate analyses were considered as potential risk factors of postoperative AA in the multivariate analyses. Log-rank and Kaplan-Meier tests were used to compare survival between groups. Multivariate analysis using Cox proportional hazards models was performed to identify whether postoperative AA development was one of the independent risk factors for mortality. All statistical analyses were carried out in SPSS version 23.0 (IBM Corp., Armonk, NY, USA). All tests were two-sided, and the results were considered statistically significant at p < 0.05.
RESULTS
Patient demographics
In total, 153 patients (age, 51.2 ± 13.6 years; men, 58.8%) who underwent LT were evaluated. Bilateral and single LT was performed in 141 (92.2%) and 12 (7.8%) patients, respectively. The major indications for LT were (in order of frequency) idiopathic pulmonary fibrosis (IPF), graft-versus-host disease after hematopoietic stem cell transplantation, lymphangioleiomyomatosis, bronchiectasis, and chronic obstructive pulmonary disease. Less frequent diagnoses included idiopathic pulmonary hypertension, miliary tuberculosis, pulmonary capillary hemangioma, Langerhans cell histiocytosis, and interstitial lung diseases associated with systemic rheumatic diseases. Comorbidities of the patients included hypertension (20.9%), diabetes (17.6%), dyslipidemia (9.8%), and coronary artery disease (7.2%). All patients had sinus rhythm at the time of surgery, but five patients (3.3%) had a history of paroxysmal AF prior to the transplant. All patients were treated with standard immunosuppressive medications, such as tacrolimus, azathioprine, and prednisolone, after surgery.
Incidence, timing, and types of AA
The mean follow-up period post-transplantation was 22.0 ± 19.1 months. During the follow-up period, AA developed in 46 of the 153 of patients (30.1%). Early postoperative AA occurred in 40 patients (26.1%) with AA, of whom 26 (17.0%) had AF, 11 (7.2%) had AFL, and three (2.0%) had AT. There were no significant differences in the onset time among patients with AF, AFL, and AT (7.2 ± 5.3, 11.3 ± 6.9, and 17.3 ± 10.3 days, respectively; p = 0.195). The occurrence of early postoperative AA peaked on the postoperative day (POD) 4. In 67.5% of patients, AA developed within POD 10, and in 90.0% of patients before POD 20 (Fig. 1). The conversion of AA into sinus rhythm was observed in all patients during hospitalization and the mean duration of early postoperative AA was 2.6 ± 2.6 days (range, 1 to 11). Late AA occurred in 11 patients (7.2%), of whom one (0.7%) had AF, two (1.3%) had AFL, and eight (5.2%) had AT. The median interval from LT to onset of late AA was 64 days (interquartile range, 45 to 95). AF and AT were the most common AA in early and late postoperative periods (65.0% and 72.7%, respectively) (Fig. 1). Conversion of late AA into sinus rhythm was also observed in all patients with a duration of 8.2 ± 10.4 days (range, 1 to 32). Five patients developed both early postoperative and late AA: three patients with early AF and one patient with early AFL developed AT: one patient with early AF developed AFL in the late postoperative period (Fig. 2) [13].
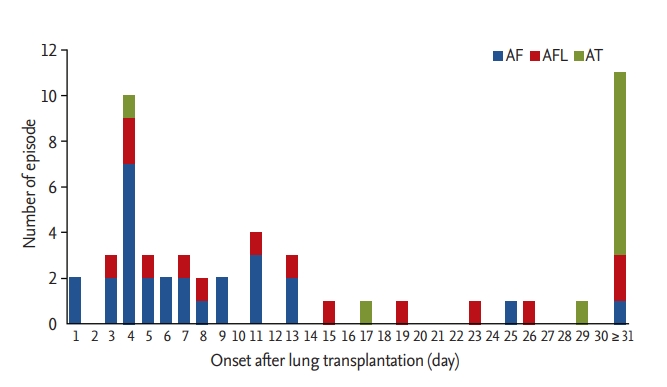
Incidence and time to onset of atrial arrhythmias after lung transplantation. AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia.
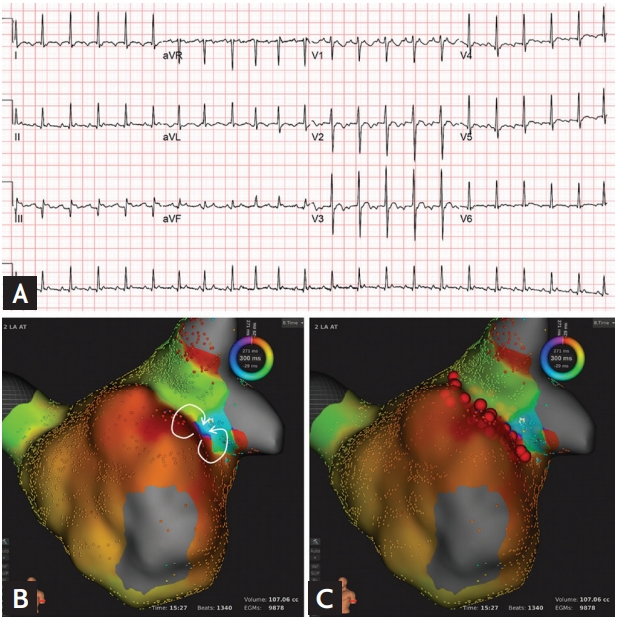
A case of a 62-year-old man who was treated for atrial tachycardia with radiofrequency catheter ablation. (A) Electrocardiography showing atrial tachycardia. (B) Three-dimensional activation map reveals figure-of-eight intra-atrial reentrant tachycardia (white arrows) with the slow conduction zone at the anastomosis line. (C) Radiofrequency catheter ablation was performed along the ridge (red balls). Adaptad from Uhm et al. [13]. aVR, augmented Vector right; aVL, augmented Vector left; aVF, augmented Vector foot.
Risk factors for AA
Differences in patients’ characteristics according to the development of AA are presented in Table 1. Patients with AA were significantly older, had larger body surface area (BSA), and had frequent incidences of AF before transplantion, IPF, and postoperative tracheostomy than those without AA. No significant differences were observed between patients with and those without AA in preoperative echocardiographic data (including LVEF, left atrial size, RAP, right ventricular systolic pressure, and E/E’), and cardiac catheterization data (including mean pulmonary artery pressure, mean right atrial pressure, and mean pulmonary capillary wedge pressure). Predictors of AA at any time after LT are shown in Table 2. Longer periods of mechanical ventilation (hazard ratio [HR], 1.03; 95% confidence interval [CI], 1.01 to 1.06; p = 0.008) and preoperative RAP (HR, 1.19; 95% CI, 1.06 to 1.35; p = 0.005) were found to be significant risk factors for AA after LT, when adjusted for other risk factors.
Treatment strategies for AA
Forty of the 46 patients (87.0%) received treatment for AA. The remaining six patients, with early postoperative AA (three AF, one AFL, and two AT), were not treated as their heart rates remained stable and the AA was spontaneously converted to sinus rhythm within 1.8 ± 1.5 days. Rate control treatment, using β-blockers, calcium channel blockers (diltiazem or verapamil), and digoxin, was used in the majority of patients (39 of 40, 97.5%). Six of the 40 patients (16.7%) were treated with combined antiarrhythmic medical therapy (amiodarone), and two patients underwent electrical cardioversion due to hemodynamic instability. Non-vitamin K-dependent oral anticoagulants were administered to five patients with an AF lasting for > 2 days and a CHA2DS2-VASc score ≥ 2.
Clinical impact of AA
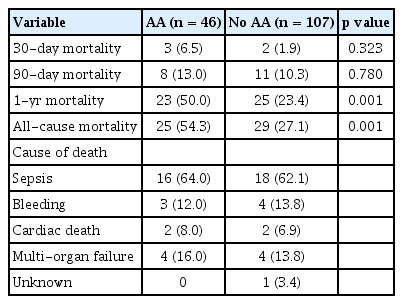
Length of ICU stay and hospital stay, as well as in-hospital mortality, were not significantly different between patients with and without AA (Table 1). Stroke developed in six patients (3.9%) after LT, and the incidence was similar between patients with and without AA. Long-term overall mortality was significantly higher in patients who developed AA in comparison to their counterparts (Fig. 3). Landmark analyses were performed to evaluate survival 30 days after LT. Incidence of all-cause mortality was higher in the patients with early postoperative AA than in those without AA (Fig. 4). Further, patients who developed late AA or both early and late AA had greater rate of all-cause mortality than those without AA. (Fig. 4).
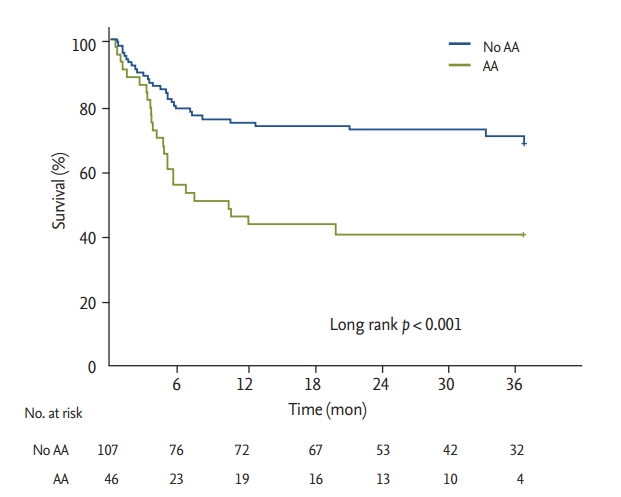
Kaplan-Meier curves for survival in patients with and without atrial arrhythmias after lung transplantation. AA, atrial arrhythmia.
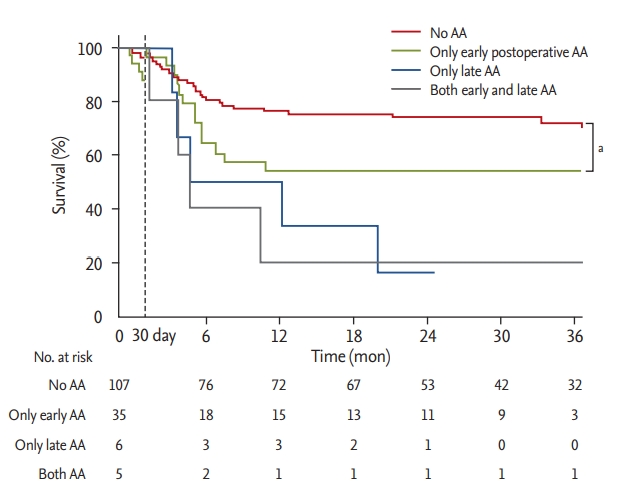
Landmark analysis for survival after the first 30 days post transplantation. AA, atrial arrhythmia. a Log lank p = 0.001.
The major causes of death in all LT recipients included infection (63.0%) and multi-organ failure syndrome (16.1%). There was no difference in causes of death between the patients with and without AA (infection: 64.0% vs. 62.1%, p = 0.872; multi-organ failure syndrome: 16.0% vs. 13.8%, p = 0.826). All-cause mortality and cause of death were summarized in Table 3.
Postoperative AA, including early and late AA, was found to be an independent predictor of overall mortality, even after adjustment for other covariates. An E/E’ > 15 and longer periods of mechanical ventilation were also identified as independent risk factors associated with increased mortality after LT (Table 4).
DISCUSSION
The major findings of this study were as follows: (1) AA was common after LT; (2) elevated preoperative RAP and long-term ventilator care were independent predictors for the development of AA after LT; and (3) all-cause mortality after LT was higher in patients with AA than in those without AA.
Potential mechanisms of AA after LT
Our data shows that AA occurred most frequently in the early postoperative period (within 30 days) and its frequency declined after 1 month. AF was the most commonly occurring AA in the early postoperative period, whereas late AA was composed of mainly AT and AFL. These observations are consistent with previously reported data [4,5,14]. AF could be occurring frequently during the early postoperative period due to local inflammation, myocardial injury, sympathetic activation, fluid shift, and electrolyte imbalances [15-18]. Additionally, the operative technique during LT, such as sutured anastomosis between the donor’s pulmonary vein cuffs and the recipient’s left atrium, can provide an electroanatomic substrate for potential AA [4,19]. However, it could also serve as an antral electrical isolation of the pulmonary veins since the suture line is similar with the line ablation created by the Cox-Maze operation and can theoretically provide an antiarrhythmogenic effect for AF in the long-term. Later occurrences of AFL or AT than AF after LT could be due to reentry from the anastomosis after healing. Azadani et al. [14] reported a greater proportion of atypical pattern of AFL in recipients who underwent LT and proposed that atypical AFL stems from the suture line of the left atrium in contrast to typical AFL, which arises from the right atrium around the tricuspid annulus. In an electrophysiological study, See et al. [4] showed cases of postoperative AT related to macro-reentry that originated from suture lines, and focal AT that arose from the pulmonary vein anastomoses. Taken together, these results suggest that surgical suture lines can help prevent AF, and can also act as substrate for the development of AFL or AT after scarring. Additional electrophysiological studies must be carried out to confirm this theory and better understand the mechanism of AA after LT.
Risk factors for AA after LT
In this study, we identified that elevated RAP and a prolonged period of mechanical ventilation period were independent predictors of AA, after adjusting for confounding variables. Mechanical ventilation induces intermittent positive pressure during respiratory cycles, which may raise intrathoracic pressure and right atrial pressure. Regardless, due to the application of additional positive end expiratory pressure, right atrial pressure may remain higher throughout the respiratory cycle [20]. Such elevated right atrial pressure is related with occurrences of AA. Furthermore, prolonged periods of mechanical ventilation can cause an increase in sympathetic activity, which may trigger the development of AA.
Clinical significance of AA after LT
Long-term all-cause mortality was significantly higher in patients with AA. Furthermore, the development of AA was identified as an independent predictor of overall mortality in the multivariate analysis. Interestingly, even though most AA occurred in the early postoperative period, early clinical outcomes, such as length of ICU stay, length of hospital stay, and in-hospital mortality, were not different between patients with and without AA in our study population. However, we clarified the prognostic impact of early postoperative AA on survival clearly in the long term via our landmark analysis. These findings are in agreement with the results reported by Orrego et al. [3], which demonstrated a higher 12-month overall mortality in those who developed AA, but not at 90 days. Despite conflicting results of the impact of postoperative AA on long term survival after LT [11,21], a recent meta-analysis demonstrated that the occurrence of AA after LT has prognostic implications for overall long-term survival [22]. Taken together, these results suggest that development of AA after LT may not influence short-term mortality, but likely affects overall long-term mortality. Moreover, we found that development of late AA was also linked with poor long-term survival after LT. To our knowledge, an association between late AA after LT and survival has not been reported in prior studies. In this study, we could not identify the differences in characteristics between patients with late AA and their counterparts due to a small sample size. Further large-scale studies need to be carried out to address this issue.
The mechanisms that drive postoperative AA and result in poor long-term survival are not known. Mean time to death after development of AA was 76.0 ± 78.5 days in our study. Most patients died from infection, bleeding or multi-organ failure, and these poor medical conditions could have acted as a trigger for AA. In addition, longer periods of perioperative mechanical ventilation, which was related to development of AA, may reflect the more complex surgery and complications like infection. Therefore, we speculate that AA may serve as a potential surrogate marker to identify unstable medical conditions that may make patients vulnerable to stress and may increase their risk of mortality.
Study limitations
There are several limitations to be noted. Given the retrospective nature of this study at a single institution with a small number of patients, unmeasured confounders associated with AA such as consistent surgical techniques or postoperative care might have biased the results despite statistical adjustments. Further, some short and asymptomatic arrhythmic episodes during inconsistent ECG monitoring in the general ward or late AA events could have been missed. However, given complete access and review of these events, it is not likely that meaningful information was lost. Additionally, we could not determine the mechanisms driving postoperative AA due to unavailable electrophysiological data in our study. Further large-scale electrophysiological studies are required to better understand the mechanisms of AA and establish prevention strategies in LT recipients.
In conclusion, patients with preoperative RAP and long-term ventilator care had a higher risk of developing AA after LT. Further, AA after LT is associated with poor long-term survival.
KEY MESSAGE
1. Atrial arrhythmia (AA) is common in patients who underwent lung transplantation (LT).
2. Elevated preoperative right atrial pressure and long-term ventilator care were associated with development of AA after LT.
3. AA after LT is linked with poor long-term survival.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.