Clinical outcomes of extracorporeal membrane oxygenation support in patients with hematologic malignancies
Article information
Abstract
Background/Aims
The clinical outcomes of patients with hematologic malignancies who were treated with extracorporeal membrane oxygenation (ECMO) after the failu re of optimal conventional therapy were determined.
Methods
The medical records of all patients administered ECMO during their stay in a medical intensive care unit of Seoul St. Mary's Hospital between February 2010 and July 2013 were reviewed retrospectively.
Results
In total, 15 patients with hematologic malignancies were compared to 33 immunocompetent patients with documented cardiorespiratory failure. Underlying hematologic malignancies were significantly associated with lower overall survival (0.0% vs. 24.2%, p = 0.044). Mortality was significantly associated with a higher 24 hours ECMO inspired fraction of oxygen (0.71 ± 0.24 vs. 0.47 ± 0.13, p = 0.015), the development of infection after ECMO (87.5% vs. 25.0%, p = 0.001), and the presence of hyperbilirubinemia (70.0% vs. 0.0%, p < 0.001). Matching of the patients based on their Acute Physiology and Chronic Health Evaluation II scores confirmed the greater risk of mortality in patients with hematologic malignancies (survival: 0.0% vs. 40.0%, p = 0.017). The mean difference in inotropic-equivalent scores after ECMO was significantly lower in the immunocompetent patients than in those with hematologic malignancies (-59.22 ± 97.83 vs. 53.87 ± 164.46, p = 0.026).
Conclusions
Patients with hematologic malignancies who require ECMO for respiratory support have poor outcomes. The incidence of complications in these patients did not significantly differ from that in immunocompetent patients.
INTRODUCTION
Recent advances in chemotherapeutic agents and hematopoietic stem cell transplantation (HSCT) have improved the prognosis of patients with hematologic malignancies [12]. Nonetheless, these patients frequently develop life-threatening complications as a result of infection, treatment, and the disease process [3456]. Complications are particularly likely to develop in the lungs and include pneumonia, pulmonary hemorrhage, bronchiolitis obliterans, and graft-versus-host disease [78]. Among the 40% of patients with hematologic malignancies who are admitted to an intensive care unit (ICU) for respiratory support, half will require mechanical ventilation [9]. However, the need for mechanical ventilation in patients with hematologic malignancies is associated with a poor prognosis [1011].
Earlier studies evaluated the outcomes of extracorporeal membrane oxygenation (ECMO) in patients with hematologic malignancies. Most of these were clinical studies involving either a mixture of patients with various types of solid tumors or pediatric patients, and the successful use of the technique in both settings was rare [121314]. The present study provides a systematic assessment of the clinical outcomes of patients with hematologic malignancies who were treated with ECMO after the failure of optimal conventional therapy, based on a retrospective review of the patients' medical records.
METHODS
The present study was based on a retrospective review of the medical records of all adult patients who underwent ECMO for cardiopulmonary support at the medical ICU of Seoul St. Mary's Hospital of The Catholic University of Korea between February 2010 and July 2013. More than 450 HSCT procedures are performed annually at this hospital. The decision to initiate ECMO is made according to the clinical judgment of the intensive care specialist after an assessment of the patient's clinical parameters. During the study period, 76 adult patients underwent ECMO at the medical ICU. From this group, 28 patients with underlying non-hematologic malignancies were excluded from the study. Thus, the baseline demographics and clinical outcomes were compared between patients with hematologic malignancies and immunocompetent controls with acute cardiorespiratory failure, all of whom underwent ECMO.
Patient demographics, disease severity scores as evaluated using the Acute Physiology and Chronic Health Evaluation (APACHE) II, lung injury score, reason for ECMO, type of ECMO, clinical parameters before and during ECMO, complications during ECMO, survival following ECMO decannulation, and the ICU- and hospital-discharge dates were analyzed based on the data available in the patients' medical records. The APACHE II score was recorded for each patient within the first 24 hours after ICU admission. Hyperbilirubinemia was defined as a total bilirubin level > 4 mg/dL, and neutropenia was defined as a neutrophil count < 500 cells/µL.
Statistical analysis
Statistical analysis was performed using Fisher exact test for discrete variables and the Mann-Whitney U test for continuous variables. A two-sided p < 0.05 indicated statistical significance. In view of the differences in disease severity between the two groups, an individualized APACHE II score-matched analysis was used to adjust for the effects of the underlying risk factors on the clinical outcomes. Each patient in the hematologic disease group was matched to a patient in the immunocompetent general disease group (1:1 match) who had the closest APACHE II score. A maximal difference of 5% in the APACHE II score was allowed in the matching process. If there was more than one possible match based on an identical APACHE II score, the patients were matched for age (first secondary-matching variable) and Glasgow coma score (backup secondary-matching variable). Matched patients were reanalyzed as described above. Survival was analyzed using the Kaplan-Meier method. All of the statistical analyses were performed using the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital (IRB no. KC13RISI0589). In view of the retrospective nature of the study, the need for informed consent from the patients was waived.
RESULTS
Demographic characteristics
The study population consisted of 15 immunocompromised patients with hematologic malignancies, and 33 immunocompetent patients with other general diseases. All of the 48 patients had received ECMO.
Hematologic malignancies
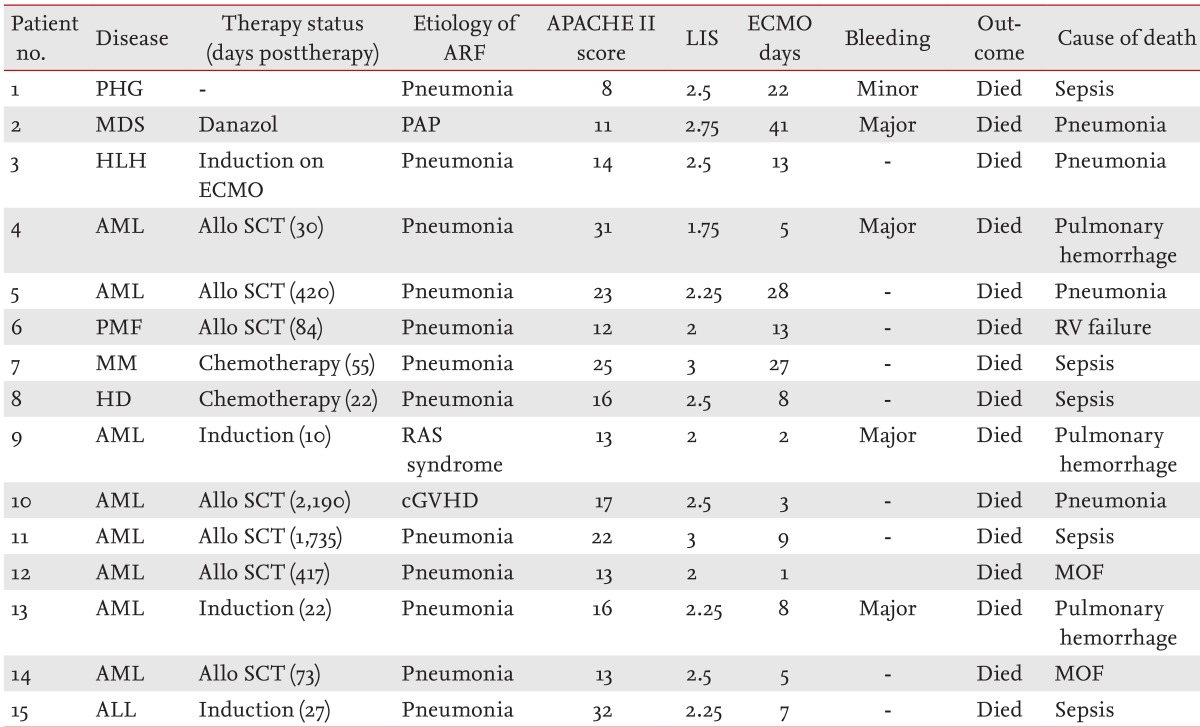
Among the patients with hematologic malignancies, 10 had leukemia, including eight with acute myelogenous leukemia and two with acute lymphoblastic leukemia. One patient each had Hodgkin lymphoma, multiple myeloma, myelodysplastic syndrome, hematophagocytic lymphohistiocytosis, and primary myelofibrosis. Five of the patients with acute myelogenous leukemia, one with primary myelofibrosis, and one with acute lymphoblastic leukemia underwent HSCT. Six of the HSCT procedures were allogeneic and one was autologous. In these patients, ECMO was indicated for pulmonary support (Table 1).
Hematologic and general disease
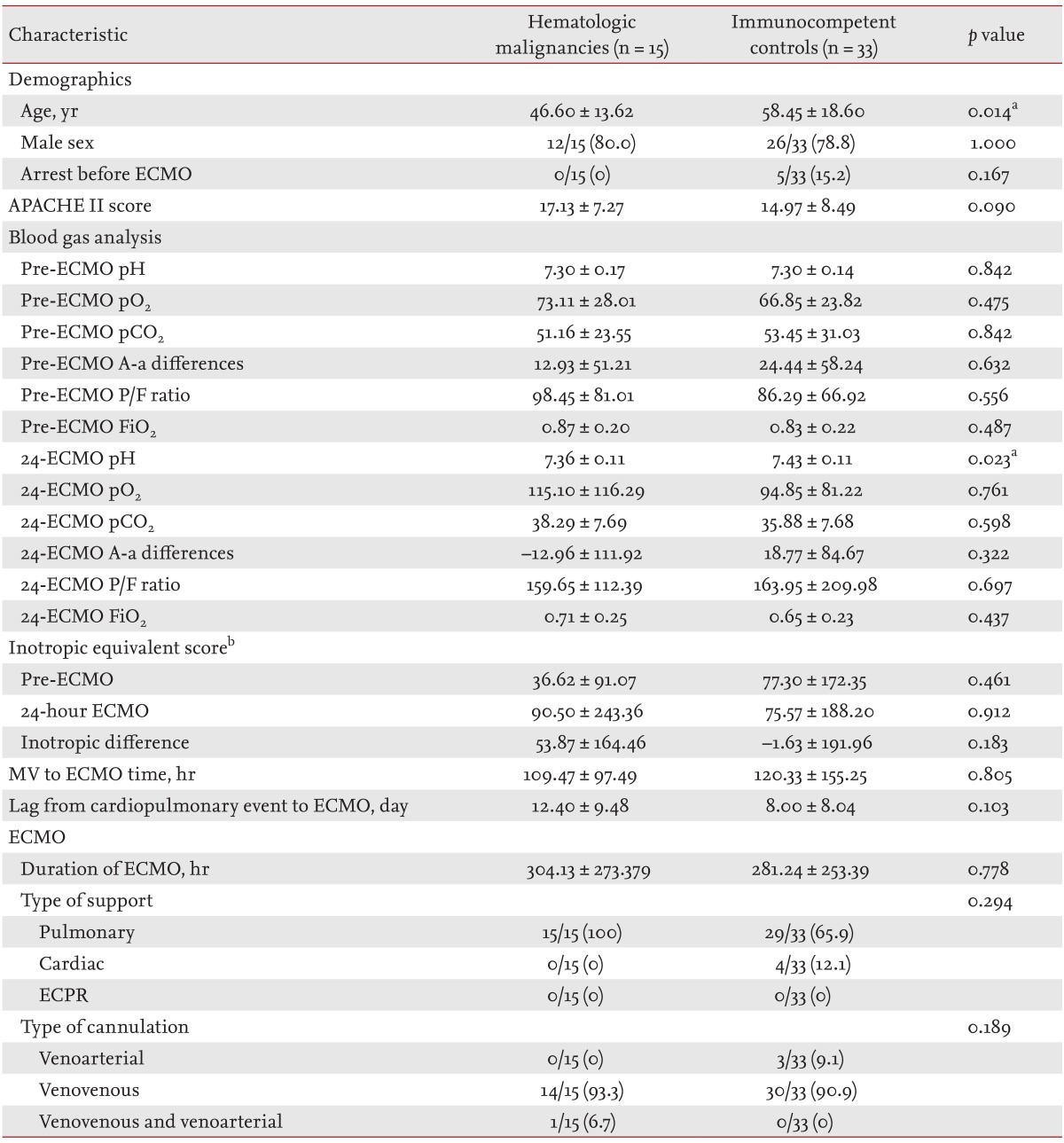
The demographics and ECMO variables of the patients with hematologic malignancies and the immunocompetent patients are compared in Table 2. The survival rate was lower in patients with hematologic malignancies (24.2% vs. 0.0%, p = 0.044). Immunocompetent patients were significantly older than patients with hematologic malignancies (58.45 ± 18.60 years vs. 46.60 ± 13.62 years, p = 0.014). The latter group had a lower mean pH after 24 hours of ECMO (7.36 ± 0.11 vs. 7.43 ± 0.11, p = 0.023). The mean duration of ECMO and the type of cannulation did not differ between the two groups.
Survivors and non-survivors
The demographics and ECMO variables of the survivors and non-survivors are compared in Table 3. Fig. 1 shows the Kaplan-Meier survival curves for the two groups. Mortality was significantly associated with underlying hematologic malignancies (0.0% vs. 37.5%, p = 0.044), the development of infection after ECMO (87.5% vs. 25.0%, p = 0.001), hyperbilirubinemia (0.0% vs. 70.0%, p < 0.001), and worse ventilation parameters after 24 hours of ECMO, especially with a higher inspired fraction of oxygen (0.71 ± 0.24 vs. 0.47 ± 0.13, p = 0.015). Inotropic-equivalent scores before ECMO were significantly higher in survivors, but after 24 hours of ECMO, they were similar because of the resulting blood pressure stabilization in those patients. The APACHE II score, pre-ECMO ventilator parameters, time between mechanical ventilation and ECMO, running time of ECMO, and types of support and cannulation were not different between the two groups.
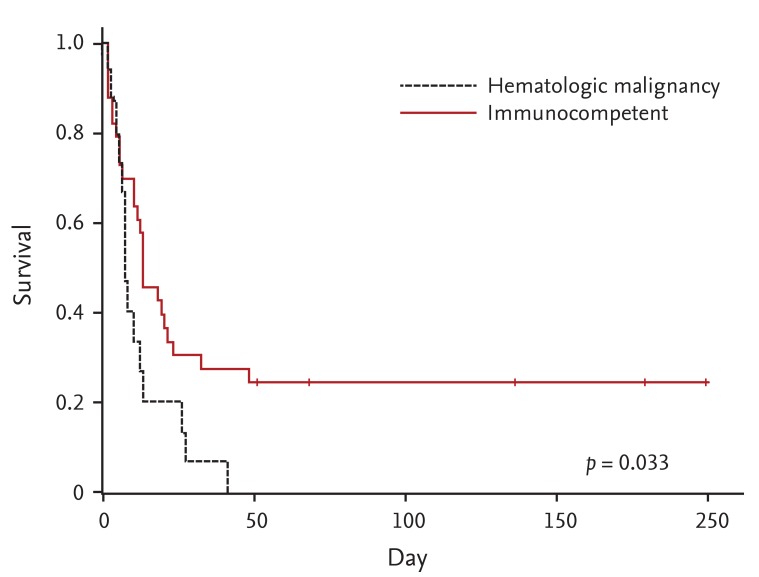
Kaplan-Meier survival analysis of patients with hematologic malignancies and immunocompetent controls matched for their Acute Physiology and Chronic Health Evaluation II scores. The survival distribution of the two groups was compared using a log-rank test. Median survival was 8.00 days versus 13.00 days, respectively.
Individualized APACHE-II-score-matched analysis
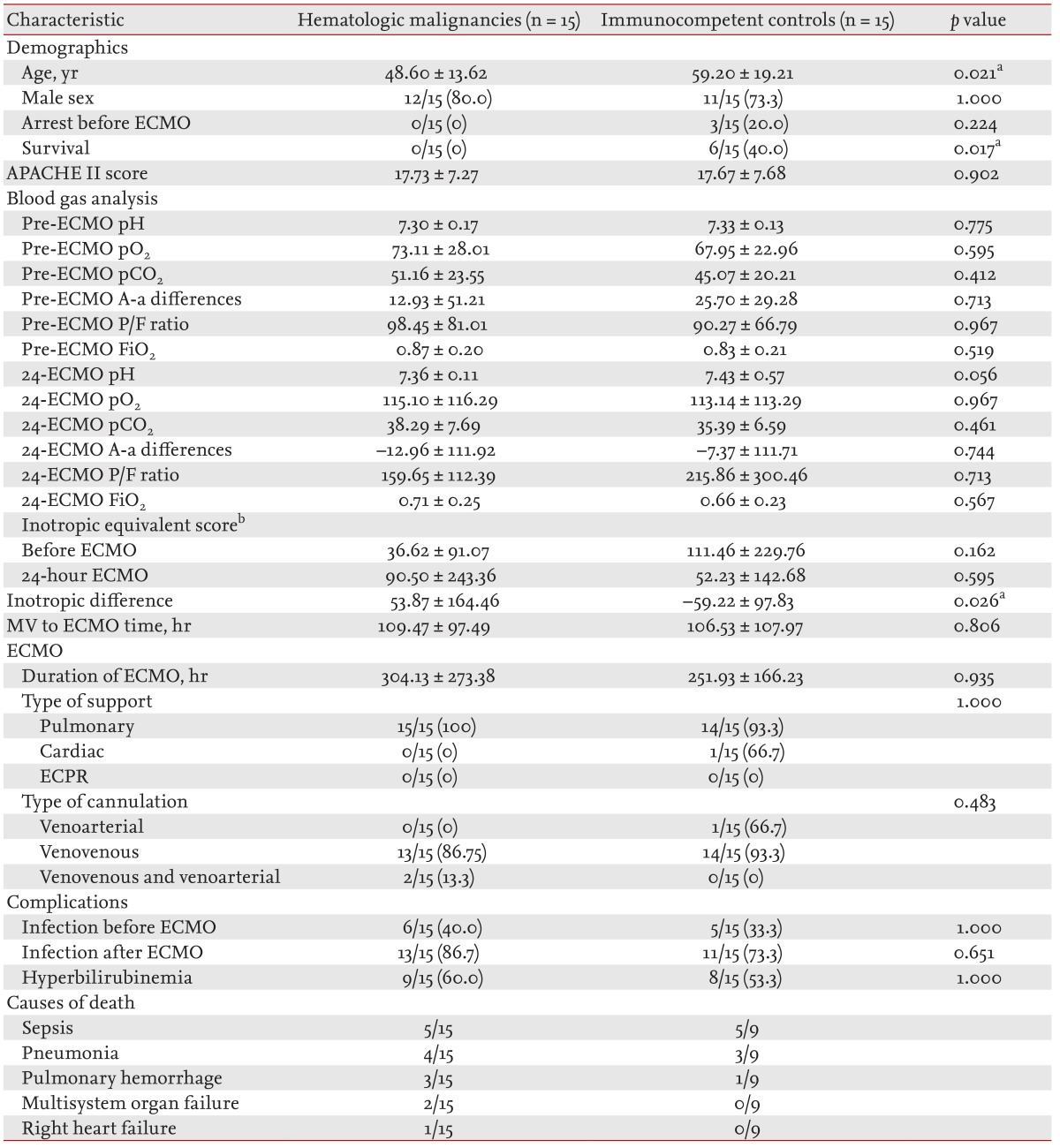
Patients in the two groups were matched based on their APACHE II scores to adjust for the effect of disease severity upon ICU admission (Table 4). After matching, mortality was higher in patients with hematologic malignancies (100.0% vs. 60.0%, p = 0.017). Inotropic-equivalent scores 24 hours after ECMO treatment were significantly lower in the immunocompetent controls (53.87 ± 164.46 vs. -59.22 ± 97.83, p = 0.026). There was no association of mortality with sex, blood gas parameters before and 24 hours after ECMO, time from mechanical ventilation to ECMO initiation, running time of ECMO, or types of support and cannulation. The causes of death (in order of occurrence) in patients with hematologic malignancies were sepsis, pneumonia, pulmonary hemorrhage, multisystem organ failure, and right heart failure; in the immunocompetent controls, they were sepsis, pneumonia, and pulmonary hemorrhage.
Complications
The reported complications were divided into hemorrhagic, neurologic, renal, cardiovascular, pulmonary, infectious, and hepatic complications, and are summarized in Table 5. Common complications were low blood pressure requiring inotropics, culture-positive infection, renal failure requiring continuous renal replacement therapy, acute renal failure (creatinine level of 1.5 to 3.0 mg/dL), and gastrointestinal hemorrhage. There were no significant differences in the incidence of complications between patients with underlying hematologic malignancies and the immunocompetent controls. A comparison of the complications in ECMO survivors and non-survivors showed a significantly higher incidence of hyperbilirubinemia and the development of infection after ECMO in the latter.
Infection
Infections before ECMO initiation were documented in 18 of the 48 patients (37.5%). The infectious agents were Acinetobacter baumannii (n = 6), Candida spp. (n = 3), Stenotrophomonas maltophilia (n = 2), Pseudomonas aeruginosa (n = 1), coagulase-negative Staphylococcus (n = 1), methicillin-sensitive Staphylococcus aureus (n = 1), methicillin-resistant S. aureus (n = 1), and Enterobacter spp. (n = 1).
Infection after the initiation of ECMO was one of the factors influencing mortality. After ECMO initiation, 37 of the 48 patients (77.0%) had documented infections; 87.5% were non-survivors and 25.0% were survivors. In total, two or more species of bacteria were isolated from 14 of these 37 patients (37.8%). The identified microorganisms were A. baumannii (n = 26), P. aeruginosa (n = 4), methicillin-resistant coagulase-negative Staphylococcus (n = 4), Candida spp. (n = 5), S. maltophilia (n = 3), Enterobacter spp. (n = 3), methicillin-resistant S. aureus (n = 1), extended-spectrum β-lactamase-producing Klebsiella pneumonia (n = 1), Serratia marcescens (n = 1), and Clostridium difficile (n = 1). The same organisms were isolated in nine of 37 patients before and after ECMO initiation; whether these were new or previous infections that were not completely treated is unknown.
DISCUSSION
Patients with hematologic malignancies frequently develop life-threatening complications as a result of infection, treatment, and the disease process [3456]. These complications often involve the lungs [7]. Chemotherapy-related pulmonary complications in patients with hematologic malignancies include pneumonia, pulmonary hemorrhage, and neoplastic infiltration, and account for the poor outcomes [15]. Early-phase complications in patients undergoing HSCT include viral, fungal, and bacterial infections and pulmonary hemorrhage, whereas common late-phase complications are bronchiolitis obliterans, bronchiolitis obliterans with organizing pneumonia, and graft-versus-host disease [816]. Acute respiratory failure is the leading cause of ICU admission in patients with hematologic malignancies, and 50% to 70% of these patients will require mechanical ventilation [917]. However, the need for mechanical ventilation is a prognostic for a poor clinical outcome [91011181920].
Most clinical studies of ECMO have either been based on mixed groups of patients with various types of solid tumors or on pediatric patients [121314]. Earlier studies evaluated the outcomes of patients with hematologic malignancies who received ECMO after the failure of conventional mechanical ventilation [212223]. However, the successful treatment of these patients was rare, which has raised ethical concerns regarding the use of ECMO in this setting. Thus, in the present study we analyzed the medical records of patients with hematologic malignancies who were treated with ECMO to determine the clinical outcomes. Our results provide relevant data for clinicians considering ECMO in patients in whom optimal conventional therapy has failed. Specifically, the outcome of ECMO was compared in patients with hematologic disease and immunocompetent patients with acute cardiorespiratory failure. Survival was higher among the immunocompetent patients than among those with hematologic malignancies. An analysis of the factors predictive of a favorable outcome after ECMO identified a reduced oxygen requirement and a reduced inotropic-equivalent score 24 hours after ECMO. Factors associated with a poor prognosis were culture-proven infection and hyperbilirubinemia during ECMO. Pre-ECMO ventilator parameters and APACHE II scores were not significantly different between survivors and non-survivors. These results suggest that the high mortality rate of patients with hematologic disease was related to the progression of organ dysfunction and infections induced by underlying conditions, such as the immunocompromised state and the disease process. Our results are consistent with previous report of the poor prognosis of pediatric patients requiring ECMO for cardiopulmonary disease after HSCT. In these patients, the development of organ failure is a predictor of mortality [12].
The APACHE II score is used to predict mortality in critically ill patients with hematologic malignancies [9]. Therefore, in the present study, it was used to match patients in the two groups and thereby reduce the selection bias caused by differences in the baseline risks for poor outcomes. After matching, the significance of hematologic malignancy as a predictor of high mortality was even greater. The inotropic-equivalent score after ECMO was significantly lower in patients with hematologic disease than in immunocompetent patients, suggesting an association between hemodynamic instability during ECMO and poor outcome. Previous study of critically ill patients with hematologic malignancies, regardless of ECMO treatment, supports our results [18].
The development of complications in our patients was associated with a poor prognosis. Patients with cardiorespiratory failure had a large number of complications during ECMO treatment. In addition, hyperbilirubinemia and the development of infection after ECMO were significantly more common in non-survivors. However, the overall incidence of complications was not significantly different between patients with hematologic malignancies and the immunocompent controls.
In critically ill patients with hematologic malignancies, the development of multiorgan dysfunction is associated with a worse prognosis [924]. Bleeding and infection, as the main complications related to ECMO, have a significant impact on mortality [2526]. Thus, underlying hematologic disease has been a relative contraindication for ECMO because of the risk of bleeding. However, our study suggests that ECMO can be performed safely even in patients with hematologic malignancies, as bleeding events were not significantly more frequent in those patients than in the controls. This finding is strongly supported by a recent study in which the incidence of bleeding complications was not higher in patients with hematologic malignancies than in control patients [27].
The present study had several limitations. First, it was a retrospective observational study. However, because there are ethical concerns about conducting clinical trials of ECMO in patients with hematologic malignancies, a prospective randomized clinical trial of the efficacy of ECMO in patients with hematologic malignancies is currently not possible. Second, this was a single-center study. Nonetheless, our institution is one of the largest centers in Asia that deals with hematologic malignancies; thus, we were able to include a relatively large number of patients who received ECMO. A further advantage was that a single protocol was followed in the treatment and management of the patients. Clinical decisions were made by a multidisciplinary team that included critical care specialists. Therefore, there was little variation in patient management and ECMO treatment strategies. Third, all of the patients with hematologic malignancies who underwent ECMO died, probably because these critically ill patients are known to have a poor prognosis [920]. However, a recent study showed a relatively good clinical outcome of patients with hematologic malignancies who were treated with ECMO [27]. A simple comparison of the outcomes in that study with those in our study is difficult because of differences in the treatment and the subsets of hematologic disease between the two study populations. For example, the majority of our patients with hematologic disease had acute leukemia, whereas in the study by Wohlfarth et al. [27], most patients had lymphoma. It is well known that patients with acute leukemia have a poorer prognosis than those with other hematologic malignancies. Further investigations of the safety of ECMO according to the subset of hematologic malignancy are needed.
In conclusion, our study showed that patients with hematologic disease who require ECMO for cardiopulmonary support have poor outcomes. Increased mortality was associated with the development of infection and hyperbilirubinemia during ECMO. However, the incidence of complications in patients with hematologic malignancy did not significantly differ from that in immunocompetent patients. Therefore, the use of ECMO in patients with hematologic disease deserves further consideration. Evidence to support ECMO as therapy in these high-risk patients must come from a well-designed randomized controlled study.
KEY MESSAGE
1. Patients with hematologic disease who require extracorporeal membrane oxygenation (ECMO) for cardiopulmonary support have poor outcomes.
2. The incidence of complications in patients with hematologic malignancy did not significantly differ from that in immunocompetent patients.
3. The use of ECMO in patients with hematologic disease in whom optimal conventional therapy failed merits further consideration.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.