Sleep quality in rheumatoid arthritis, and its association with disease activity in a Korean population
Article information
Abstract
Background/Aims
The aim of this study was to compare the sleep quality between rheumatoid arthritis (RA) patients and healthy controls; and to evaluate the relationship between RA disease activity and sleep quality in Korea.
Methods
A total of 130 RA patients and 67 age- and sex-matched healthy controls were enrolled in a comparative study of sleep quality using the Pittsburgh Sleep Quality Index (PSQI). Age, gender, concomitant medication, erythrocyte sedimentation rate, serum C-reactive protein, Beck Depression Inventory second edition (BDI-II), 28 joints disease activity score (DAS28), pain visual analog scale (VAS), and PSQI were analyzed as covariates. We also analyzed the sleep quality of RA patients according to the disease activity (DAS28 ≤ 3.2, 3.2 < DAS28 < 5.1, and DAS28 ≥ 5.1, respectively).
Results
The total PSQI score and the frequency of poor sleep quality, were higher in the RA patients (5.62 ± 4.19, 38.5%) than in the control subjects (3.57 ± 2.17, 13.4%). The patients with poor sleep quality (PSQI > 5) were older and had a higher BDI-II and VAS score than the patients without sleep disturbance (PSQI ≤ 5). The score in subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, daytime dysfunction, total PSQI, and frequency of poor sleep quality were increased when RA activity was high.
Conclusions
Sleep disturbance was observed in RA patients (38.5%), and high RA disease activity was associated with poor sleep quality in Korea.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease characterized by joint pain, joint swelling, fatigue, sleep disturbance, and functional disability [1,2,3]. RA patients complain of various articular as well as extra-articular symptoms. Sleep disturbance is one of the most particular concerns in RA patients [2,3]. It was shown that poor sleep quality was higher in RA patients than the normal population, and sleep problems and their related symptoms occurred in 54% to 70% of RA patients [4]. Possible causes of sleep disturbance in RA patients include pain, mood, and disease activity [5].
Some studies have found a relationship between disease activity and sleep problems [6,7,8,9]. A recent study performed structural equation modeling (SEM) to identify potential indirect effects to explain the relationship between disease activity and sleep quality [6]. Pain indirectly affected sleep quality through disease activity. Biologic agents are often used in RA patients who do not respond to conventional treatments, and dramatic improvements have been shown in clinical symptoms and signs. Moreover, biologic agents have been reported to improve sleep quality in RA patients [10,11]. However, the relationship between sleep quality and RA disease activity are still poorly understood.
Few studies of sleep disturbance in RA have been conducted among Koreans. A study conducted on 97 RA patients reported a positive association between pain and sleep disturbance [12]. Another study on 119 RA patients showed that 71% of patients had sleep disturbance [13]. However, the above-mentioned studies had some limitations, in that disease activity was not used as a factor influencing sleep quality. In addition, the methods for sleep quality were not valuable and appropriate for RA.
We therefore aimed to compare sleep quality between RA patients and healthy controls, and to investigate the relationship between RA disease activity and sleep quality in a Korean population.
METHODS
Study population
The study participants were enrolled from August 2011 to February 2012, among RA patients who were registered with the rheumatology clinic of Keimyung University Dongsan Medical Center in Daegu. A total of 130 patients were of Korean nationality, and met the 1987 American College of Rheumatology criteria for RA [14]. We also recruited 67 age- and sex-matched healthy subjects as the control group. Inclusion criteria included age ≥ 18 and regular treatment included conventional and biologic disease modifying anti-rheumatic durg (DMARD) for 3 months prior to study entry. Exclusion criteria were serious comorbid medical conditions, a past history of serious psychiatric disorders, or substance abuse. This study had a cross-sectional design.
Assessment of clinical variables
Questionnaires were given to all RA patients and healthy controls, with detailed instructions and demonstrations. In case of RA, the disease duration, the erythrocyte sedimentation rate (ESR), the serum C-reactive protein (CRP), concomitant treatment (corticosteroid, nonsteroidal anti-inflammatory drugs, DMARDs, or anti-tumor necrosis factor [anti-TNF]), tender-swollen joint count, and visual analog scale (VAS) of patient's pain were evaluated.
Assessment of RA disease activity
The 28 joint disease activity score (DAS28) was used to assess RA disease activity [15]. The DAS28 consists of the number of tender and swollen joint counts (0 to 28), ESR, and patient global score (0 to 100). The subjects were categorized according to the distribution of disease activity level as remission/low disease activity (DAS28 ≤ 3.2), moderate disease activity (3.2 < DAS28 < 5.1), or high disease activity (DAS28 ≥ 5.1).
Assessment of sleep quality
We evaluated sleep quality of patients and the healthy controls using the Korean version of the Pittsburg Sleep Quality Index (PSQI) [16]. The PSQI measured the patient's reported sleep quality over the preceding month. The PSQI has 19 items measuring the following seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. A PSQI score > 5, based on the total score (0 to 21), was defined as the cutoff for a diagnosis of insomnia.
Assessment of depression
We evaluated depression of patients using the Korean version of the Beck depression inventory second edition (BDI-II) [17]. The BDI-II measured patient's reported depression according to the answers to 21 questions. The score ranged from 0 to 63, with higher scores indicating the presence of more symptomatology. A score of 13 or greater was recommended as the cutoff for depression.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). Results were presented as means ± SDs unless otherwise specified. The Mann-Whitney test was used for quantitative variables, and the chi-square or Fisher exact test was used for qualitative variables to compare RA patients with healthy controls. The nonparametric Kruskal-Wallis test and linear by linear association were used for between-group comparisons according to disease activity. The Bonferroni correction was applied to multiple comparisons within each category of variables. Spearman's correlation coefficients were used to examine the relationships between the PSQI, BDI-II, and DAS28. Multiple regression analysis was performed to detect the risk factors for the occurrence of sleep disturbance. When p values were less than 0.05, results were considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (IRB No. 2011-266). Written informed consent was obtained from all participants and confirmed by the board.
RESULTS
Demographic characteristics and the PSQI of RA patients and the healthy controls
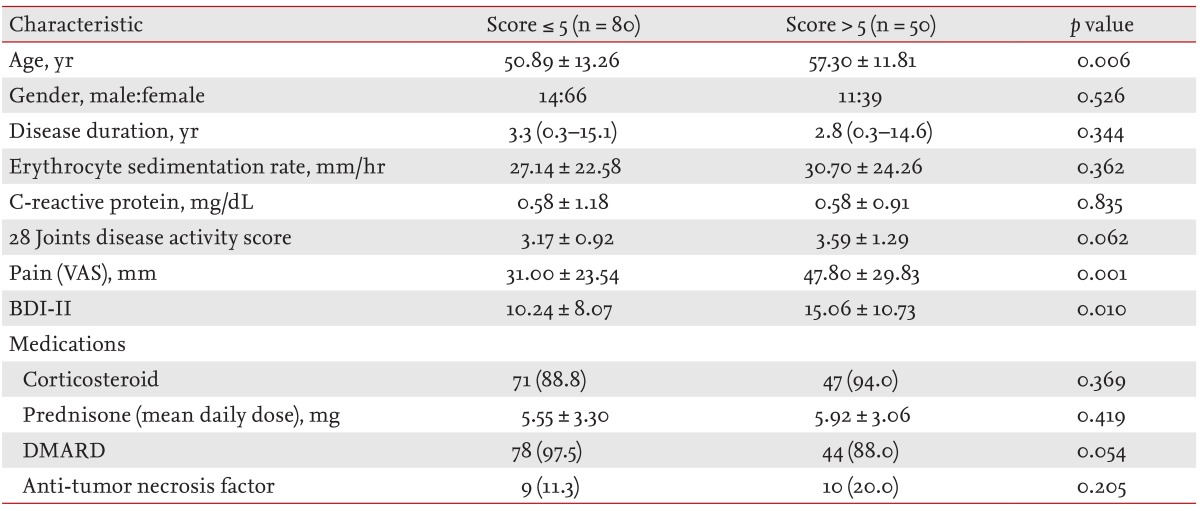
The demographic and clinical characteristics of the study subjects and healthy controls were summarized in Table 1. Mean ± SD DAS28, pain VAS, BDI-II, and total PSQI in the RA patients was 3.33 ± 1.09, 37.46 ± 27.29, 33.09 ± 9.44, 5.62 ± 4.19, respectively. The RA patients had statistically higher scores in subjective sleep quality, sleep duration, and habitual sleep efficiency domains and the total PSQI score compared to the healthy controls (p < 0.05) (Table 2). The frequency of poor sleep quality (PSQI > 5) was much more in RA patients (38.5%) than in the healthy controls (13.4%, p < 0.05).
The comparisons of RA patients with and without poor sleep quality
The demographic and clinical characteristics of RA patients with and without poor sleep quality were listed in Table 3. The patients with poor sleep quality were older and had higher BDI-II and VAS score than the patients without sleep disturbance.
The relationship of patient's characteristics, sleep disturbance and depression with RA activity
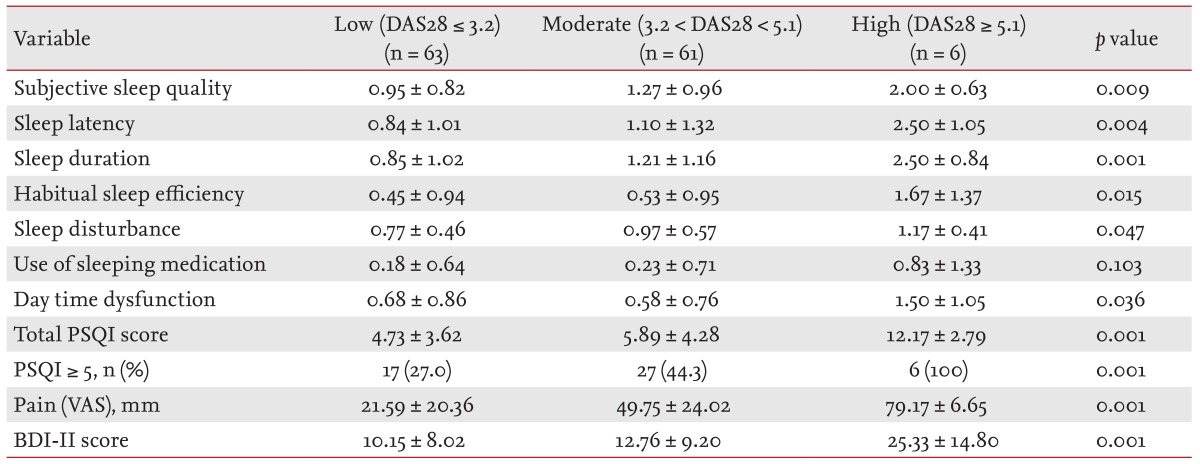
The 130 RA patients in the present study consisted of 48.5% patients in remission/low disease activity (63/130), 46.9% with moderate disease activity (61/130), and 4.6% with high disease activity (6/130). There was no statistical significance in all demographic and clinical variables except pain VAS, BDI-II, and PSQI (data not shown). High disease activity group had significantly higher pain VAS and BDI-II score than remission/low and moderate disease activity groups (Table 4). The score in subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, daytime dysfunction, and total PSQI was increased when RA activity was high. The frequency of poor sleep quality was also associated with the disease activity (p = 0.023).
Correlation and regression analysis
According to Spearman's analysis, there was a significantly higher correlation between DAS28 and BDI-II (r = 0.375, p = 0.001), between PSQI and BDI-II (r = 0.300, p = 0.001). However, there was no association between sleep disturbance and age, gender, corticosteroids, DMARDs, anti-TNF use, ESR, CRP, BDI-II, DAS28, and pain VAS in multivariate regression analysis (data not shown).
DISCUSSION
Similar to Western populations, the prevalence of insomnia disorder is 5% in the general population of South Korea [18]. Currently, the frequency of poor sleep quality is higher in RA patients than in normal population [4,5]. Our data corroborated previous findings that RA patients had poor sleep quality compared with healthy controls in Korea, based on results of the total PSQI and the ratio of high PSQI score (> 5), although polysomnography was not used to measure the objective sleep quality [19].
The present study showed that the frequency of poor sleep quality was higher in RA patients with high disease activity than in patients with low disease activity in Korea, as shown by the recent studies [4,6]. All demographic and clinical variables, such as age, gender, corticosteroids, DMARDs, anti-TNF use, ESR, CRP, BDI-II, DAS28, and pain VAS, were analyzed for their relationship with sleep quality. Age, pain VAS, and BDI-II were significantly associated with sleep quality in univariate analysis, but not in multivariate analysis (data not shown).
The recommendations of treating RA are targeted to goals of reaching remission or low disease activity to maximize long term health-related quality of life through control of symptoms, prevention of structural damage, normalization of function, and social participation [20]. The reciprocal association between pain, fatigue, depression, and disability may be suggested. Poor sleep quality may cause pain and other associated symptoms, and the pain may affect quality of life and sleep quality [2]. In future, serial measurements of sleep quality should be prospectively investigated to compare between sleep quality and disease activity.
In this study, there were no associations between sleep quality and the RA medications, including corticosteroid, DMARDs, and anti-TNF. Because anti-TNF users comprised only nine subjects (14.6%), it was difficult to suggest definitively that anti-TNF had no relationship with the sleep quality. The association between sleep quality and anti-TNF is still unclear. However, some studies showed that anti-TNF and other biologics increased the sleep quality [10,11,21,22,23], although the methodology of the studies was fairly different.
Depression is one of the most common psychological disorders. A high frequency of depression in RA patients has been reported (19% to 42%) [24,25]. One study conducted in Korea reported that 54 (50.9%) out of 106 RA patients suffered from depression [13]. In the present study, the frequency of depression in RA patients was 33.8% (44/130). This difference in frequency likely resulted from the use of different questionnaires [13]. The pain VAS and the ratio of female were associated with depression in univariate analysis. However, there was no relationship between depression and various variables on multivariate regression analyses (data not shown).
The PSQI in this study was used to evaluate the sleep quality in RA. The PSQI is commonly used for sleep quality in various diseases or health status worldwide [26]. OMRACT 9 (9th International Consensus Conference on Outcome Measures in Rheumatology Clinical Trials) reviewed instruments assessing sleep quality in RA patients according to feasibility and psychometric properties, and Athens Insomnia Scale (AIS) and Medical Outcome Study (MOS) Sleep Measure were highly ranked [27]. Both AIS and MOS Sleep Measure were not available because there was no proper Korean validation of the questionnaires. Interest has increased in sleep quality in RA patients; hence, the development of the Korean version of these instruments is needed.
Our study had some limitations. As mentioned above, the patients and controls were not evaluated according to objective changes by polysomnography. We did not perform SEM to identify potential indirect effects for the explanation of relationship between disease activity and sleep quality, as shown by Westhovens et al. [6]. The present study was also tested by cross-sectional design. However, as far as we know, this is the first study using statistical analysis to evaluate correlation of RA disease activity and the sleep quality in Korea. We think that this study will facilitate interest in sleep problems in RA patients. Future prospective studies are needed to reveal associations between disease activity and sleep quality.
In conclusion, the frequency of poor sleep quality was 38.5% in RA patients, and conversely, high RA disease activity was also associated with poor sleep quality in a Korean population. Therefore, physicians should be aware of this interaction in the treatment of RA.
KEY MESSAGE
Poor sleep quality was frequently found in Korean patients with rheumatoid arthritis (RA, 38.5%).
High RA disease activity was associated with poor sleep quality in a Korean population.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.