Treatment of BK virus-associated hemorrhagic cystitis with low-dose intravenous cidofovir in patients undergoing allogeneic hematopoietic cell transplantation
Article information
Abstract
Background/Aims
BK virus (BKV) has been associated with late-onset hemorrhagic cystitis (HC) in recipients of hematopoietic stem cell transplantation (HSCT). Cidofovir has been used at higher doses (3 to 5 mg/kg/wk) with probenecid prophylaxis; however, cidofovir may result in nephrotoxicity or cytopenia at high doses.
Methods
Allogeneic HSCT recipients with BKV-associated HC are treated with 1 mg/kg intravenous cidofovir weekly at our institution. A microbiological response was defined as at least a one log reduction in urinary BKV viral load, and a clinical response was defined as improvement in symptoms and stability or reduction in cystitis grade.
Results
Eight patients received a median of 4 weekly (range, 2 to 11) doses of cidofovir. HC occurred a median 69 days (range, 16 to 311) after allogeneic HSCT. A clinical response was detected in 7/8 patients (86%), and 4/5 (80%) had a measurable microbiological response. One patient died of uncontrolled graft-versus-host disease; therefore, we could not measure the clinical response to HC treatment. One microbiological non-responder had a stable BKV viral load with clinical improvement. Only three patients showed transient grade 2 serum creatinine toxicities, which resolved after completion of concomitant calcineurin inhibitor treatment.
Conclusions
Weekly intravenous low-dose cidofovir without probenecid appears to be a safe and effective treatment option for patients with BKV-associated HC.
INTRODUCTION
Hemorrhagic cystitis (HC) is often a serious complication of hematopoietic stem cell transplantation (HSCT), with the potential to cause significant morbidity, and is associated with prolonged hospitalization [1,2,3,4,5]. Known predisposing factors include allogeneic HSCT, conditioning regimen, advanced age at transplantation, possible graft-versus-host disease (GVHD), thrombocytopenia, coagulopathy, and viral infection [4,5]. HC is classified into two types: (1) early onset, occurring within 48 to 72 hours after HSCT, and usually due to the toxic effects of the conditioning chemotherapy, including cyclophosphamide and busulfan; and (2) late-onset, occurring after 72 hours, and usually with an infectious cause, particularly BK virus (BKV), and most frequently associated with late-onset HC [6,7,8].
Treatment of HC varies dependinag on severity. Supportive measures, including painkillers, forced diuresis, hyperhydration, continuous bladder irrigation, and transfusions, have been the standard of care for many years. Clinicians have used intravenous or intravesical cidofovir for severe cases of BKV-associated HC [2,5,8,9,10,11,12,13]. Cidofovir is a cytosine derivative of an acyclic nucleoside phosphonate analog with broad-spectrum activity against many DNA viruses, including cytomegalovirus virus (CMV), adenoviruses, and polyomaviruses [14,15]. Cidofovir has been used at higher doses (3 to 5 mg/kg/week) for prophylaxis with probenecid; however, high doses of cidofovir may result in nephrotoxicity or cytopenia [5]. Low-dose intravenous cidofovir (0.25 to 1.5 mg/kg) or prophylaxis with probenecid has been successfully used to treat BKV-associated HC without nephrotoxicity [5,9].
No standard dosage of cidofovir has been established for BKV-associated HC. Here, we report the effects of low-dose intravenous cidofovir without probenecid prophylaxis for treatment of BKV-associated HC.
METHODS
This retrospective clinical trial was conducted at Chonnam National University Hwasun Hospital, Korea, and was approved by the hospital Institutional Review Board. Patients were diagnosed with BKV-associated HC after allogeneic HSCT, and those patients who received low-dose cidofovir treatment were selected for this study. Patients diagnosed with BKV-associated HC who improved with supportive treatment alone were excluded. The diagnosis of HC was made according to the presence of clinically significant hematuria along with dysuria and/or lower abdominal pain. The severity of HC was graded according to published criteria: grade 1, microscopic hematuria with urinary symptoms; grade 2, macroscopic hematuria; grade 3, macroscopic hematuria with colts and/or decreased hemoglobin levels requiring transfusions; grade 4, life-threatening bleeding, unresponsive to treatment [1,9]. We checked urine and plasma BKV viral titers if HC was suspected in a patient who received allogeneic HSCT. A total of 178 patients received allogeneic HSCT, and 16 were diagnosed with BKV-associated HC between 2009 and 2013. Eight patients did not receive cidofovir, because they had grade 1 to 2 HC and improved after vigorous supportive care alone. Thus, eight patients were enrolled in this study.
All patients received hyperhydration, bladder irrigation using a Foley catheter, and 400 mg/day fluoroquinolone to reduce the BKV viral load [16]. Patients were treated concomitantly with intravesical hyaluronic acid at a dose of 40 mg in 50 mL of normal saline via a Foley catheter for 20 minutes each week. The patients received intravenous cidofovir (1 mg/kg) in 100 mL of normal saline weekly without probenecid prophylaxis until their clinical symptoms improved [9].
Urine and plasma BKV viral loads were quantified using commercially available Real-Q BKV quantification kits (Biosewoom Inc., Seoul, Korea). Serum creatinine and cell blood counts were closely monitored at least every 3 days.
Clinical response was defined as a subjective improvement in symptoms and stability or reduction in the grade of cystitis. Microbiological response was defined as at least a one log reduction in BKV viral load [9]. Nephrotoxicity was defined as a ≥ 0.3 mg/dL increase in serum creatinine at any time during and up to 2 weeks after cidofovir treatment.
We retrospectively reviewed medical records for basal hematological disease, conditioning regimen, donor type, associated GVHD, and concomitant immunosuppressant use at the time BKV-associated HC was diagnosed. Acute GVHD was defined according to the modified Seattle criteria, and chronic GVHD was evaluated using the National Institutes of Health consensus report [17,18].
RESULTS
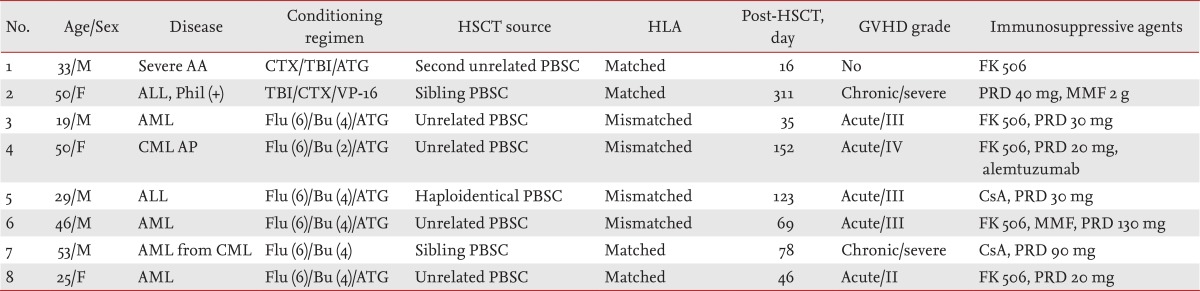
Eight patients (five males and three females; mean age, 33 years) were diagnosed with BKV-associated HC and received low-dose intravenous cidofovir from 2009 to 2013 (Table 1). The underlying hematological disease was acute leukemia in six patients (67%) and chronic myeloid leukemia (CML) with accelerated phase and severe aplastic anemia in two patients. All patients received allogeneic HSCT from various donors: two patients from sibling donors, five from unrelated donors, and one from a haploidentical family donor. Seven patients had acute or chronic GVHD at the time of the HC diagnosis and had taken moderate to high doses of glucocorticoid and immunosuppressive agents. One other patient without GVHD underwent a second HSCT. HC was diagnosed at a median interval of 69 days (range, 16 to 311) after allogeneic HSCT. The severity of HC was scored as grade 2 in four patients (50%) and grade 3 in the other four patients (50%).
Weekly cidofovir (1 mg/kg) was administered to all patients intravenously. The patients received a median of four doses of cidofovir (range, 2 to 19 doses). Two patients received only two doses of cidofovir; one showed a clinical response after only two doses, and the other received only two doses because of a deteriorated condition resulting from uncontrolled GVHD and pneumonia. The median duration of symptoms was 28 days (range, 10 to 54) (Table 2). Seven patients showed improved symptoms and urinary findings, and thus successful control of HC. The other patient with accelerated-phase CML showed non-remission after allogeneic HSCT. We stopped FK506 treatment on day 24, after which the patient developed grade 4 acute GVHD. She received immunosuppressive agents to control acute GVHD. After allogeneic HSCT at day 141, severe lung and liver chronic GVHD and CMV viremia were observed, and HC developed on day 151. She died of pneumonia and chronic GVHD on day 196 after HSCT, and we could not measure the clinical or microbiological improvement of HC after cidofovir treatment. Follow-up urine BKV viral titer was available for five of the eight patients. Four patients demonstrated a microbiological response, and one patient had a stable BKV viral load with clinical improvement. Of the other three patients, two displayed clinical improvement after four cycles of cidofovir. All patients had BKV viremia at diagnosis of BKV-associated HC. Only four patients were available for follow-up of plasma BKV titers after cidofovir therapy, and all showed microbiological improvement.
Three of eight patients showed higher creatinine levels (> 0.3 mg/dL) than those at the start of cidofovir treatment; however, all of them concomitantly received calcineurin inhibitors and recovered after completing treatment with the immunosuppressive agents. One patient (no. 7) developed severe thrombocytopenia (18,000/µL) and recovered after controlling chronic GVHD.
DISCUSSION
Here, we report the effects of low-dose cidofovir without prophylaxis using probenecid for controlling BKV-associated HC. Eight patients were diagnosed with BKV-associated HC and were treated with low-dose intravenous cidofovir. Clinical improvement was observed in seven of the eight patients, but one died of chronic GVHD regardless of HC. Microbiological improvement in urine was demonstrated in four of five patients.
BKV is a human polyomavirus acquired during childhood [19]. The seroprevalence of BKV has reached 90% in children, and chronic carriage in the genitourinary tract follows the first-infection in childhood [20,21]. Patients who present with disease after BKV reactivation are typically transplant recipients under immunosuppressive treatment [20]. Cidofovir is an effective antiviral agent administered to control BKV-associated HC in patients who have received allogeneic HSCT [5,9,22,23]. Treatment of BKV-associated HC generally depends on the severity of the HC. To our knowledge, no controlled studies have assessed the clinical response rate after only supportive care according to disease severity. Gaziev et al. [12] compared the efficacy of cidofovir treatment with that of supportive care alone among 30 pediatric patients with HC. Both groups showed a complete clinical response, but patients with low-grade HC were more frequently assigned to the supportive care groups [12]. The majority of patients enrolled in these studies had HC of grade 2 or higher and received cidofovir [2,5,12]. All of our patients had undergone intensive immunosuppression because of associated GVHD, and all exhibited HC of grade 2 or higher. Most patients received high-dose glucocorticoids with other immunosuppressive agent(s) for control of GVHD, and another patient without GVHD underwent a second HSCT. In these cases, we did not expect spontaneous resolution of HC with only supportive care.
Cidofovir is a nucleotide analog of cytosine that forms cidofovir phosphocholine, an analog of cytidine 5-diphosphocholine, within cells. Cidofovir phosphocholine is speculated to interfere with the normal synthesis and/or degradation of membrane phospholipids, resulting in proximal tubular injury and, in extreme cases, cell necrosis [24]. Proximal tubular cells express an organic anion transporter that efficiently transports various acyclic nucleotide analogs [24]. Administration of probenecid, which competes with cidofovir for the organic anion transporter and reduces proximal tubular uptake of these analogs, decreasing nephrotoxicity [24]. In several studies, 5 mg/kg of cidofovir was commonly used, and patients routinely received probenecid to prevent nephrotoxicity. Various rates of nephrotoxicity ranging from 0% to 30% have been reported at this dose [2,5,12]. Some reports have shown successful resolution of HC by maintaining intravesical levels of cidofovir to prevent renal toxicity [5,10,22,23]. Savona et al. [9] reported successful outcomes with low-dose cidofovir without probenecid; clinical and microbiological response rates were 84% and 47% in 19 patients, respectively. All of our patients received the same dose of intravenous cidofovir; however, most patients received reduced-intensity conditioning with anti-thymocyte globulin, and the stem cell sources were peripheral blood stem cells. Thus, they were at high risk of GVHD, and six of eight patients actually showed severe acute or chronic GVHD. All patients in our study achieved clinical improvement. We were unable to evaluate the clinical response in only one patient because of uncontrolled GVHD and pneumonia. We reconfirmed the effects of low-dose cidofovir even under severely immunocompromised conditions. Three patients (38%) developed a transient elevation of serum creatinine level; however, they concomitantly received immunosuppressive agents to control GVHD. It appears that low-dose cidofovir without probenecid may achieve a higher urinary concentration and is accompanied by less nephrotoxicity because of the low systemic dosage. In general, up to 70% of patients undergoing allogeneic HSCT show elevated serum creatinine levels during treatment with calcineurin inhibitors [25]. All of our patients exhibited improved serum creatinine levels after discontinuing the immunosuppressive drugs. The renal toxicity associated with low-dose cidofovir was tolerable and reversible although the patients received concomitant calcineurin inhibitors. The previously reported treatment results and toxicities of intravenous cidofovir are summarized in Table 3 [2,5,9,12,22,26,27].
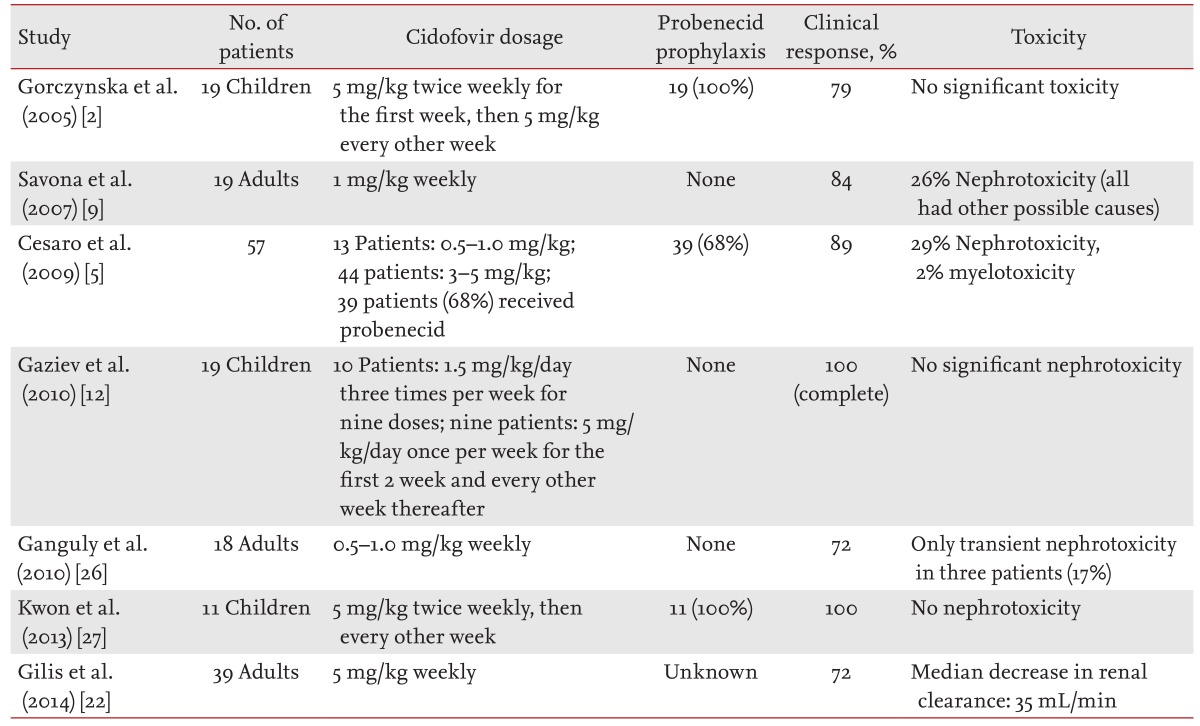
Literature review of intravenous cidofovir use for BK virus-associated hemorrhagic cystitis after allogeneic stem cell transplantation
Gilis et al. [22] showed that patients with BKV viremia of > 104 copies/mL have a significantly higher risk of developing BKV-associated HC. In our study, five of eight patients (63%) had high plasma viral titers (≥ 104 copies/mL). Among them, three were available for follow-up plasma BKV titer measurement, and all showed decreased viral titers of < 104 copies/mL after low-dose cidofovir therapy. The number of patients included in this study was too small to determine the role of cidofovir in BKV viremia. However, we assumed that cidofovir might have contributed to the microbiological improvement in viremia. Furthermore, low-dose intravenous cidofovir reduces complications and precludes the use of probenecid, making it a cost-effective treatment [22].
Despite good clinical outcomes, this study had some limitations with regard to generalization of the efficacy and safety of low-dose intravenous cidofovir in patients with BKV-associated HC. First, this was a retrospective analysis, and a small number of patients from a single institute were enrolled. In addition, not all of the patients underwent a methodical serial follow-up of their BKV viruria and viremia. However, because no standard dose of cidofovir has been established for BKV-associated HC and limited clinical data are available regarding the effectiveness of low-dose intravenous cidofovir and toxicity, we have provided evidence that low-dose intravenous cidofovir is an effective and safe modality for controlling of BKV-associated HC.
In conclusion, weekly intravenous low-dose cidofovir appears to be a safe and effective treatment for BKV-associated HC because it may reduce the renal toxicity normally caused by a standard cidofovir dose in these patients.
KEY MESSAGE
Weekly intravenous low-dose cidofovir was shown to be an effective, safe treatment for BK virus-associated hemorrhagic cystitis.
Weekly intravenous administration of low-dose cidofovir precludes the use of probenecid, making it a cost-effective treatment.
Notes
No potential conflict of interest relevant to this article was reported.