The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes
Article information
Abstract
Background/Aims
Recent epidemiological studies revealed a striking inverse relationship between vitamin D levels, glucose intolerance/insulin resistance (IR), and cardiovascular disease. However, few interventional studies have evaluated the effect of vitamin D supplementation on cardiovascular risk, such as IR and arterial stiffness, in diabetes. We investigated the role of vitamin D supplementation on cardiovascular risk in type 2 diabetes patients, including metabolic parameters, IR, and arterial stiffness.
Methods
We enrolled patients who were taking antidiabetic medications or managed their diabetes using lifestyle changes. We excluded patients who were taking vitamin D or calcium supplements. We randomized participants into the vitamin D group (cholecalciferol 2,000 IU/day + calcium 200 mg/day, n = 40) or the placebo group (calcium 200 mg/day, n = 41). We compared their IR (homeostasis model of assessment [HOMA]-IR) and arterial stiffness (brachial-ankle pulse wave velocity and radial augmentation index) before and after 24 weeks of intervention.
Results
The baseline characteristics of the two groups were similar. A total of 62 participants (placebo, 30; vitamin D, 32) completed the study protocol. At the end of the study period, the 25-hydroxyvitamin D [25(OH)D] levels were significantly higher in the vitamin D group than in the placebo group (35.4 ± 8.5 ng/mL vs. 18.4 ± 7.3 ng/mL, p < 0.001). There was no difference in HOMA-IR or changes in arterial stiffness (placebo, 21, vitamin D, 24) between the groups.
Conclusions
Our data suggest that high-dose vitamin D supplementation might be effective in terms of elevating 25(OH)D levels. However, we identified no beneficial effects on cardiovascular risk in type 2 diabetes, including IR and arterial stiffness.
INTRODUCTION
Vitamin D functions as a hormone to regulate bone metabolism via calcium homeostasis. The recent renewed interest in vitamin D results from a worsening trend of worldwide deficiency as well as novel insights regarding its effects on glucose metabolism [1,2], endothelial function [3,4], and the cardiovascular system [5,6,7,8].
Nearly half of Koreans are vitamin D-deficient [9], which is cyclically aggravated in seasons with low physical activity, such as winter and spring. Obesity, including a higher body mass index (BMI), waist circumference, total fat mass, and percentage of fat mass, is also a major risk factor for vitamin D deficiency [10]. In patients with type 2 diabetes, the prevalence of vitamin D deficiency is almost twice that in nondiabetic individuals [11].
Vitamin D levels are negatively related to insulin resistance (IR) [12] and arterial stiffness [13,14]. Diabetes combined with a vitamin D-deficient state doubles the relative risk of developing cardiovascular disease and mortality compared with diabetes with normal vitamin D levels [15,16]. IR might link vitamin D deficiency and arterial stiffness in type 2 diabetes. Several interventional trials were performed to evaluate the effect of vitamin D supplementation on IR [17,18,19], endothelial function [20,21,22], and arterial stiffness [23] in nondiabetic, insulin-resistant subjects. One interventional study evaluated the effect of vitamin D on endothelial function in patients with type 2 diabetes [24]. However, no interventional trials have evaluated the effect of vitamin D on both IR and arterial stiffness in type 2 diabetes.
Therefore, we investigated the effect of high-dose vitamin D3 (cholecalciferol) supplementation on IR and arterial stiffness in vitamin D-deficient patients with type 2 diabetes.
METHODS
Subjects
This study was a prospective, randomized, double-blinded, placebo-controlled trial in type 2 diabetic patients with 25-hydroxyvitamin D [25(OH)D] concentrations < 20 ng/mL during the screening period (from November 2011 to January 2012) in Korea. We enrolled participants whose glycemic control was stable and who could continue physical activity during the winter. The inclusion criteria were as follows: (1) ambulatory participants aged 30 to 69 years; (2) hemoglobin A1c (HbA1c) levels 6.5% to 8.4%; (3) unchanged medication (antidiabetic drugs, antihypertensive drugs, antilipid drugs, and antiplatelet drugs) within 3 months before the study; (4) normal calcium levels; and (5) BMI ≥ 23 kg/m2. The exclusion criteria were: (1) the use of osteoporosis-related medications (estrogen, selective estrogen receptor modulator, bisphosphonate, vitamin D, or calcium) within 3 months before study; (2) the use of insulin within 1 month before study; (3) systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 100 mmHg; (4) acute myocardial infarction or stroke within 6 months; (5) abnormal liver function test (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] ≥ 2.5-fold the upper normal limit); or (6) alcoholic individuals (weekly alcohol consumption ≥ 140 g). The study was performed at the Hallym University Chuncheon Sacred Heart Hospital in Chuncheon, Korea. The Institutional Review Board of Hallym University Chuncheon Sacred Heart Hospital approved the study protocol. All subjects provided written informed consent. This trial was registered in clinicaltrial.gov (ClinicalTrials.gov number, NCT01854463). It was also approved by the Korea Food and Drug Administration (KFDA) because the approved daily dose of oral vitamin D3 in Korea is < 1,000 IU (KFDA-11866).
Study design
The addition of 1,000 IU of vitamin D3 daily could be expected to increase circulating 25(OH)D levels by ~10 ng/mL [25,26]. In our previous pilot study, the 25(OH)D levels of Korean type 2 diabetes mellitus (DM) patients were 8.8 ± 3.2 ng/mL in winter (February) and 16.0 ± 6.0 ng/mL in summer (August) [27]. The recent guidelines recommend an optimal level of 25(OH)D of > 30 ng/mL [28]. Therefore, we selected 2,000 IU of vitamin D3 as the daily supplemental dose for study subjects to achieve a target level of 25(OH)D.
The participants were randomized to receive either 1,000 IU of cholecalciferol (inactive vitamin D3, Dalim BioTech, Hwaseong, Korea) combined with 100 mg of elemental calcium (vitamin D group) twice daily or a placebo containing 100 mg of elemental calcium twice daily (placebo group) for 24 weeks within 1 month of screening. The vitamin D and placebo tablets were identical in size, shape, color, and ingredients except for the cholecalciferol. The randomization was carried out at a 1:1 ratio without blocking using computer-generated random numbers. The randomization code was kept in a sealed envelope until after the last visit of the last participant. A physical examination and blood chemistry were performed in all of the participants at baseline, at 12 ± 2 weeks and 24 ± 4 weeks. We also evaluated the daytime outdoor physical activity and arterial stiffness of all patients at baseline and at the end of the study. All participants were instructed not to change their previous lifestyle, including outdoor physical activity and sun exposure. We educated participants regarding vitamin D-rich diets. Their physicians did not change their antihypertensive, antiplatelet, and antilipid medications, which affect arterial stiffness, during the study period. The patients' physicians were permitted change their antidiabetic drugs according to their glycemic state.
Outcome measures
In this study, we evaluated metabolic parameters (fasting glucose, lipid profiles, HbA1c, insulin, the homeostasis model of assessment-IR [HOMA-IR], high-sensitivity C-reactive protein [hsCRP], brachial-ankle pulse wave velocity [baPWV], radial augmentation index [AIx], and central systolic blood pressure [cSBP]). We also evaluated the safety profile of high-dose vitamin D supplementation in terms of serum calcium, liver, and kidney function.
Anthropometric and biochemical analysis
Patient body weight and height were measured with the subjects wearing light clothing without shoes. Waist circumference was measured from the narrowest point between the lower borders of the rib cage and the iliac crest. BMI was calculated as the weight in kilograms divided by the height in meters squared. Blood pressure was measured in the sitting position after a 10-minute rest period (peripheral brachial blood pressure).
Blood samples were drawn after an overnight fast and were centrifuged immediately. Levels of serum calcium, phosphorus, creatinine, AST, ALT, lipid profiles, and HbA1c were measured in an overnight fasting state from 7:00 to 9:00 AM. Serum 25(OH)D was measured using a direct competitive chemiluminescence immunoassay (DiaSorin Liaison, Stillwater, MN, USA). Insulin was measured using an electrochemiluminescence immunoassay method (Roche, Mannheim, Germany). HOMA-IR was calculated using the following formula: fasting glucose (mg/dL) × fasting insulin (µU/mL) / 405 [29]. The serum intact parathyroid hormone (PTH) level was determined using a chemiluminescence immunoassay (reference value, 15 to 65 pg/mL) and that of hsCRP was measured using turbidimetry (Beckman Coulter, Miami, FL, USA).
Measurement of arterial stiffness and central blood pressure
Arterial stiffness was evaluated using the PWV and heart rate corrected AIx. The subject was examined in the supine position in a temperature-controlled room for 10 minutes before PWV measurements. The PWV was measured using a waveform analyzer (model VP-2000, Colin, Komaki, Japan). The baPWV was calculated as the mean of the measured right baPWV (right upper arm-right ankle) and left baPWV (left upper arm-left ankle). After the PWV examinations, the arterial pulse waveforms of the left radial artery were measured noninvasively using an automated tonometric system (HEM-9000AI, Omron Healthcare Co., Kyoto, Japan) after 10 minutes of rest in a sitting position. Pulse wave analyses were performed at least twice and the mean was analyzed. Radial arterial waveforms from the first (P1) and late (second) systolic peaks (P2) were identified automatically using the fourth derivative wave as the second and third zero crossing points, respectively. The AIx. was defined as the ratio of the height of P2 to that of P1. This system estimates cSBP from the P2 value using linear regression. All AIx. measurements were read independently and were standardized to a heart rate of 75 bpm. One trained technician performed all measurements.
Statistical analysis
The statistical analyses were performed using STATA 12 (Stata Statistical Software, College Station, TX, USA). The results are expressed as mean ± standard deviation (SD) or number (%). Data with skewed distributions are expressed as medians and interquartile ranges. Comparisons between groups were performed using two-tailed Student t tests. Categorical variables were compared by chi-square test. Statistical analyses were performed after logarithmic transformation in skewed distributions. We analyzed the data of participants with good compliance, who took the study drugs > 80% of the time and did not change their antidiabetic, antilipid, antihypertensive, or antiplatelet agents. A p < 0.05 was considered to indicate significance.
RESULTS
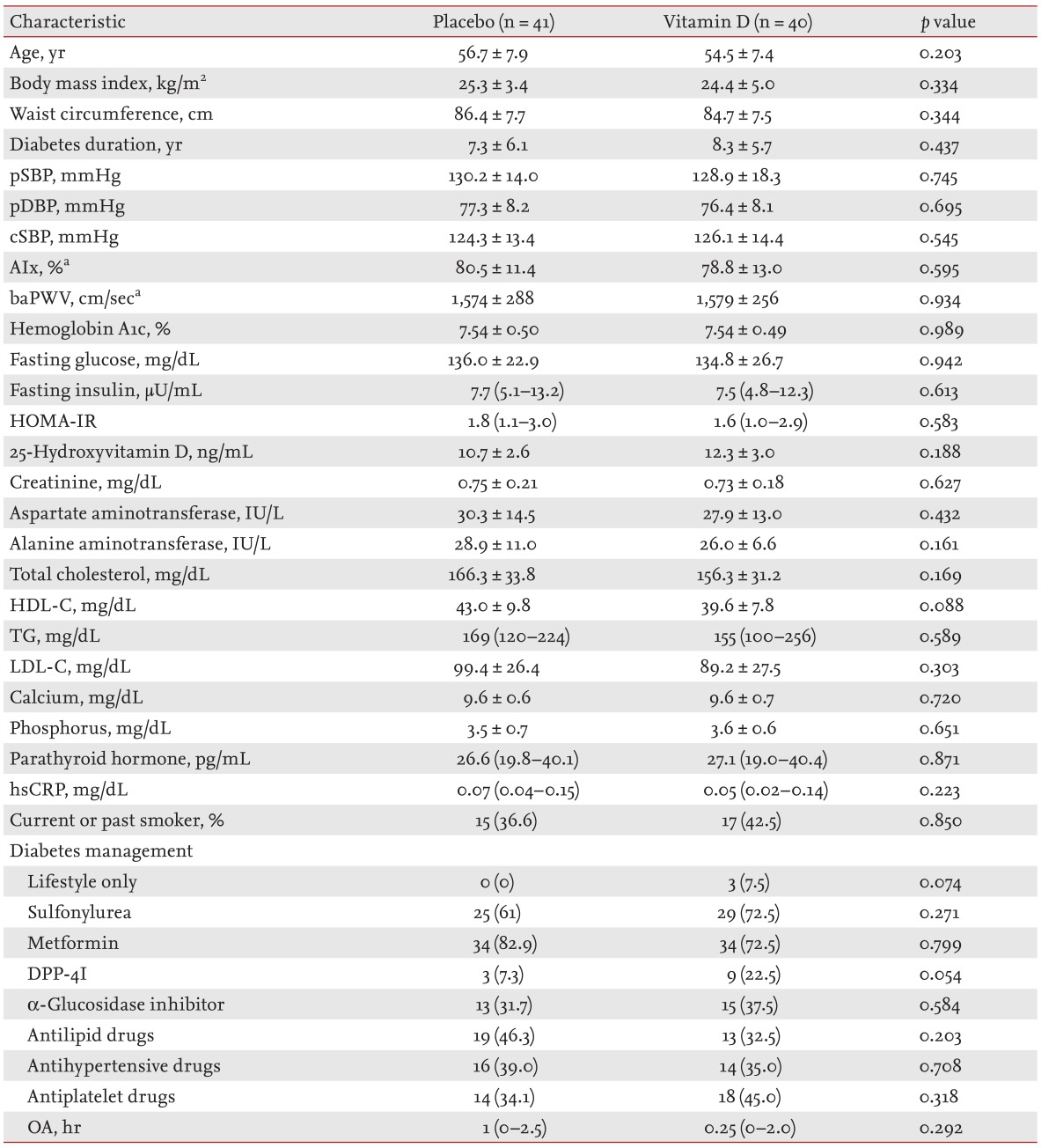
Fig. 1 shows a flow diagram of the trial. Of the 90 participants who were screened, 81 were randomized into the placebo (n = 41) and vitamin D groups (n = 40). There were no differences between the groups in terms of age (56.7 ± 7.9 years vs. 54.5 ± 7.4 years, p = 0.437), diabetes duration (7.3 ± 6.1 years vs. 8.3 ± 5.7 years, p = 0.437), medications (antidiabetic, antihypertensive, antihyperlipidemic, and antiplatelet agents), smoking status, and daytime outdoor physical activity level. In addition, the glycemic status (HbA1c, 7.54% ± 0.50% vs. 7.50% ± 0.49%, p = 0.989), 25(OH)D levels (10.7 ± 2.6 ng/mL vs. 12.3 ± 3.0 ng/mL, p = 0.188), HOMA-IR, lipid profiles, and hsCRP were similar between the groups. Only one participant had PTH levels > 65 ng/mL. Baseline cSBP, baPWV, and AIx were similar between the groups (placebo group, n = 33; vitamin D group, n = 38) (Table 1).
The data of 62 participants (30 and 32 from the placebo and vitamin D groups, respectively) were analyzed because some patients withdrew, changed their medications (antidiabetic, antihypertensive, antilipid, and antiplatelet drugs), or had poor compliance (< 80% intake of the study medication). At the end of the study, 25(OH)D levels reached 35.4 ± 8.5 and 18.4 ± 7.3 ng/mL in the vitamin D and placebo groups, respectively (p < 0.001). There were also significant differences in the adequacy of the vitamin D level (≥ 30 ng/mL). In the placebo group, 10% (n = 3) had adequate levels of vitamin D, compared with 68.8% (n = 22) in the vitamin D group (p < 0.001). There were no differences in changes in HbA1c, HOMA-IR, lipid profiles, hsCRP, and PTH between groups. There were also no differences in AIx (ΔAIx, -4.3% ± 7.8% vs. -2.2% ± 9.4%, p = 0.399), baPWV (ΔbaPWV, -60 ± 176 cm/sec vs. -16 ± 137 cm/sec, p = 0.348), and cSBP between the placebo (n = 21) and vitamin D (n = 24) groups (Table 2). Similarly, there were no significant changes in baPWV or AIx after adjusting for age, DM duration, and pulse pressure before and after the study (Fig. 2). During the trial, no adverse effects related to calcium metabolism or renal and hepatic function occurred.
DISCUSSION
In this study, we evaluated the effect of 24-week, high-dose (2,000 IU/day) vitamin D supplementation in 25(OH)D-deficient type 2 diabetes patients. High-dose vitamin D supplementation was very safe and effective at elevating 25(OH)D levels. Nearly 70% of the participants reached the target levels of 25(OH)D in the vitamin D-treated group, compared with only 10% in the placebo group during summer. Nevertheless, there was no beneficial effect of high-dose vitamin D supplementation on cardiovascular risk factors-including glycemic control, HOMA-IR, lipid profiles, hsCRP, cSBP, baPWV, AIx, or PTH levels-in this 24-week trial.
Vitamin D deficiency is more prevalent and severe in patients with type 2 diabetes compared with the normal population [11]. Epidemiological studies revealed that vitamin D deficiency accompanied by type 2 diabetes is associated with an increased risk of all-cause and cardiovascular mortality [15,16]. Vitamin D receptors are found in several tissue types throughout the body, including vascular smooth muscle [30], the endothelium [31], and cardiomyocytes [32]. In many observational studies, vitamin D deficiency was strongly associated with cardiometabolic risk factors such as endothelial dysfunction [3] and diabetes [1,2]. Vitamin D was suggested to negatively regulate the renin-angiotensin system [33,34] and inhibit smooth muscle cell proliferation and macrophage activation [35,36].
IR is associated with arterial stiffness and cardiovascular disease independent of glucose tolerance status and hypertension [37,38]. Arterial stiffness is a powerful independent marker of cardiovascular disease in diabetic patients [39]. IR might also link arterial stiffness and vitamin D deficiency in type 2 diabetes. The role of vitamin D in glucose metabolism is to increase insulin secretion from pancreatic β-cells and increase insulin sensitivity in peripheral target tissues [40]. Several interventional trials were performed to evaluate the effect of vitamin D supplementation in nondiabetic insulin-resistant subjects [17,18,19]. Insulin sensitivity and IR were improved significantly and fasting insulin was decreased after daily supplementation with 4,000 IU of daily vitamin D in insulin-resistant Asian females compared with a placebo control. IR was improved when the endpoint serum 25(OH)D reached ≥ 32 ng/mL [17]. However, in other trials there was no improvement in glucose metabolism, insulin secretion, or inflammatory markers despite 25(OH)D levels approaching 70 ng/mL [18,19]. There was no amelioration in glycemic control, lipid profiles, or HOMA-IR in the current study.
Vitamin D supplementation improved flow-mediated vasodilation (FMD) in several randomized trials [4,20,21,22]. However, other studies reported no beneficial effects on endothelial function [41,42,43] and arterial stiffness [23] in nondiabetic subjects. In the current study, vitamin D supplementation had no beneficial effects on arterial stiffness (PWV and AIx) in type 2 diabetic patients.
The elevated PTH levels in the vitamin D-deficient state might aggravate endothelial dysfunction. The inverse association between vitamin D and PWV might be dependent on serum PTH levels. Pirro et al. [44] showed that elevated PTH levels in vitamin D-insufficient, postmenopausal females were a significant predictor of aortic stiffness, irrespective of cardiovascular risk factors. Yiu et al. [24] recently reported the results of very high-dose vitamin D (5,000 IU/day) supplementation for 12 weeks in patients with type 2 diabetes with suboptimal vitamin D (< 30 ng/mL) levels. Although the PTH levels were decreased in the intervention group compared with the placebo group, vitamin D supplementation did not improve vascular function, as determined by FMD or baPWV [24]. All of the participants in the current study were vitamin D-deficient (< 20 ng/mL). However, most of the participants had PTH levels within the normal range. After high-dose vitamin D supplementation for 24 weeks, the vitamin D state was improved significantly in the vitamin D-treated group. However, unlike the results reported by Yiu et al. [24], there were no differences of PTH levels. Aloia et al. [45] studied the threshold of vitamin D levels that increased PTH levels. The threshold was 16.8 ng/mL (42 nmol/L), and 25(OH)D levels above this point did not show clinical or statistically significant PTH changes [45]. At the end of the current study, the mean vitamin D level was 18.4 ng/mL in the placebo group. The nonsignificant changes in PTH between groups might be due to higher levels of vitamin D during the summer in the placebo group than the threshold. In the current study, vitamin D supplementation had no beneficial effect on PTH levels, which might explain the lack of improvement in arterial stiffness (baPWV and Aix).
Increased arterial stiffness is affected primarily by blood pressure, which causes repetitive pulsatile stress on the arterial wall and leads to both structural and functional changes in the artery. Arterial stiffness is also increased in metabolic syndrome and insulin-resistant states [46]. In the current study, there was no improvement in any of the components of metabolic syndrome, including glycemic control (HbA1c), lipid profiles, or blood pressure after vitamin D supplementation. There were also no effects on IR (HOMA-IR), inflammatory marker (hsCRP), or PTH in the vitamin D group. The lack of reduction in arterial stiffness might have arisen from the negative effects of vitamin D supplementation on these arterial stiffness-related cardiovascular risk factors. The intervention period might also have been of insufficient duration to make any structural or functional improvement in the arteries.
This study had several strengths over other similar works. We did not change the patients' medications, including antidiabetic, antihypertensive, antilipid, or antiplatelet medications, as doing so could have affected the results. In addition, all of the participants were vitamin D-deficient. However, this study had several potential limitations. First, the results might have been confounded by the coadministration of calcium because the KFDA has approved only compounds that combine oral vitamin D3 and calcium. Excess calcium in the serum might have caused pathological changes by influencing calcification modulators such as pyrophosphate and by binding to calcium-sensing receptors on vascular smooth muscle cells [47]. Second, the current study included relatively few participants. As such, our study did not have sufficient power to detect small differences in metabolic parameters, HOMA-IR, or arterial stiffness between groups. Third, the baseline PTH levels in the majority of participants were normal, which suggests that the vitamin D deficiency in the study participants was not severe. It is also possible that the dose of vitamin D used was insufficient to change PTH levels significantly, which might have affected arterial stiffness. Fourth, this study started during winter and ended in summer, 6 months later. During this period, the gradual increase in serum 25(OH)D levels due to the increased sun exposure might have reduced the effect of vitamin D supplementation, reducing the differences between the groups.
High-dose vitamin D supplementation in vitamin D-deficient patients with type 2 diabetes might be effective and safe at elevating 25(OH)D levels. However, we found no beneficial effects on IR or arterial stiffness. Additional long-term, high-dose, interventional trials in type 2 diabetic patients with severe vitamin D-deficiency should be performed to evaluate the effects of vitamin D supplementation on cardiovascular risk factors, such as IR and arterial stiffness.
KEY MESSAGE
Vitamin D levels are negatively related to insulin resistance (IR) and arterial stiffness. IR might link vitamin D deficiency and arterial stiffness in type 2 diabetic patients. However, no interventional trials of the effect of vitamin D on both IR and arterial stiffness in type 2 diabetes have been performed.
High-dose vitamin D supplementation had no beneficial effects on cardiovascular risk factors (glycemic control, lipid profiles, IR, blood pressure) or arterial stiffness (brachial-ankle pulse wave velocity and radial augmentation index) in vitamin D-deficient type 2 diabetic patients.
Acknowledgments
This work was supported by a grant from Hallym University Medical Center Research Fund (01-2010-02).
Notes
No potential conflict of interest relevant to this article was reported.