Successful treatment with tacrolimus of refractory adult-onset Still's disease
Article information
To the Editor,
Adult-onset Still's disease (AOSD) is a systemic inflammatory disorder characterized by high fever, arthralgia, transient rash, hepatosplenomegaly, lymphadenopathy, liver dysfunction and leukocytosis. The diagnosis of AOSD is conferred based on clinical findings after elimination of other causes. The mainstay of treatment is glucocorticoids and/or nonsteroidal anti-inflammatory drugs, although most cases are refractory to these conventional therapies. To treat steroid-resistant AOSD, previous reports have suggested the use of immunosuppressants such as methotrexate (MTX), cyclosporine A (Cy-A), tumor necrosis factor-alpha (TNF-α) blockers, an interleukin (IL)-1 blocker and an IL-6 receptor blocker. Recent studies suggested that higher levels of IL-18 are detected in patients with active AOSD, which correlate with disease activity and inflammatory features. In addition, AOSD with high levels of IL-18 is refractory to treatment and is often steroid-resistant. A successful outcome was reported recently in one case of refractory AOSD treated with tacrolimus (Tac), a calcineurin inhibitor similar to Cy-A [1]. Here, we report a case of AOSD with high IL-18 levels that was treated unsuccessfully with steroid in combination with Cy-A, but was subsequently treated successfully using steroid in combination with Tac.
A 58-year-old female was admitted to our hospital with a 2-week history of fever, polyarthralgia, myalgia, sore throat, and erythematous rash. Physical examination revealed the following: body temperature of 38.7℃; erythematous plaques and papules on the face, trunk, arms and extremities; enlarged cervical lymph nodes; and throat redness. Her skin rashes persisted with color tones that faded slightly with falling fever, but the rashes were not evanescent. The laboratory findings were as follows: white blood cell count (WBC), 24,520/mm3 (neutrophils, 91%; eosinophils, 3.0%; monocytes, 4.0%; lymphocytes, 2.0%); red blood cell count, 349 × 104/mm3; hemoglobin, 10.3 g/dL; platelet count, 25.8 × 104/mm3; aspartate aminotransferase, 88 IU/L; alanine aminotransferase, 29 IU/L; total protein, 5.5 g/dL; albumin, 2.6 g/dL; IgG, 916 mg/dL; IgM, 73 mg/dL; IgA, 159 mg/dL; rheumatoid factor, < 15 IU/mL; C3, 166 mg/dL; C4, 32 mg/dL; and C-reactive protein (CRP), 11.80 mg/dL (normal range, < 0.3 mg/dL). Immune complexes were not detected by the C1q-binding assay. The antinuclear antibody (ANA) titer was < 40×. All tests were negative for other autoimmune antibodies, including anti-ds-DNA, anti-SS-A, anti-SS-B, anti-DNA, anti-ribonucleic protein, anti-Smith, anti-cardiolipin, anti-centromere, anti-topoisomerase, and anti-mitochondria antibodies, and were negative for the myeloperoxidase and proteinase-3 antineutrophil cytoplasmic antibodies. Urinalysis revealed neither proteinuria nor hematuria. Computed tomography revealed bilateral axillary lymphadenopathy, slight left pleural effusion and slight splenomegaly. The diagnosis of AOSD was established based on the presence of pyrexia, sore throat, arthralgia, lymphadenopathy, leukocytosis, liver dysfunction, the lack of rheumatoid factor and the negative ANA titer. The clinical findings met three of the four major criteria and four of the four minor criteria for AOSD classification proposed by Yamaguchi et al. [2]. Treatment with prednisolone (PSL) at 50 mg/day was initiated; 2 days later, the admission levels of ferritin were reported to be 28,728 ng/mL (normal range, 5 to 157 ng/mL); therefore, PSL was exchanged for methyl-PSL at 1 g/day for 3 days (Fig. 1). Subsequently, treatment consisted of 50 mg/day PSL in combination with 200 mg/day Cy-A. Five days after the first methyl-PSL pulse therapy, the fever improved, with the WBC and CRP decreasing to 15,160/mm3 and 1.47 mg/dL, respectively. However, 10 days after the initial methyl-PSL pulse therapy, the fever reappeared, with WBC and CRP increasing to 20,320/mm3 and 3.54 mg/dL, respectively. Retreatment with methyl-PSL at 1 g/day for 3 days was performed, followed by administration of PSL at a maintenance dose of 50 mg/day in combination with Cy-A at 200 mg/day. At that time, the ferritin level was 1,814 ng/mL. The trough and 2-hour peak levels of Cy-A were 110 and 3,625 ng/mL, respectively; therefore, the Cy-A dosage was reduced to 150 mg/day. The fever improved immediately, with WBC and CRP levels decreasing to 12,270/mm3 and 0.61 mg/dL, respectively, 6 days after the second methyl-PSL pulse therapy. However, a slight fever reappeared 2 weeks after beginning that regimen, with WBC and CRP levels increasing to 20,660/mm3 and 0.75 mg/dL, respectively. Because 50 mg/day PSL was administered for ~4 weeks, the dosage could not be increased. Therefore, the third pulse therapy regimen of methyl-PSL at 1 g/day for 3 days was administered, followed by administration of PSL at 40 mg/day in combination with Cy-A at 150 mg/day. The fever improved immediately, with WBC and CRP decreasing to 10,100/mm3 and 0.2 mg/dL, respectively. At this time, the ferritin level was 1,010 ng/mL. However, a slight fever reappeared 3 weeks after the third methyl-PSL pulse therapy, with WBC of 7,350/mm3 and CRP increasing to 0.42 mg/dL. Because the IL-18 and IL-6 levels measured just before the second methyl-PSL pulse therapy were 89,645 pg/mL (normal range, < 260 pg/mL) and 11 pg/mL (normal range, < 4 pg/mL), respectively, and ferritin increased again to 1,793 ng/mL, Tac at 2 mg/day was initiated as an alternative to Cy-A. The trough level of Tac was 2.3 ng/mL, so the dosage was increased to 3 mg/day. The fever improved, and CRP was found to be negative. A normal body temperature and negative CRP levels continued. Ferritin decreased to normal levels (148 ng/mL) 3 weeks after initiation of Tac treatment, and the IL-18 level decreased gradually to 1,437 pg/mL after 5 months. The PSL dose was reduced gradually from 50 to 10 mg/day over a 5-month period, and the patient was discharged.
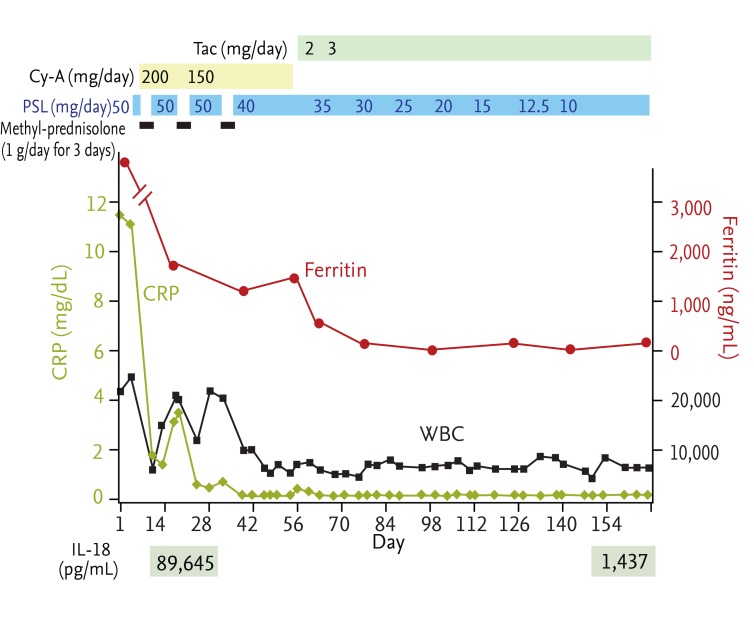
Clinical course. Cyclosporine A (Cy-A) in combination with glucocorticoid (prednisolone [PSL] or methyl-PSL) was administered. Subsequently, Tacrolimus (Tac) (2 mg/day) was initiated, and the dose of Tac was increased to 3 mg/day. Both C-reactive protein (CRP) and ferritin levels were completely normalized by Tac even after Cy-A was discontinued. WBC, white blood cells.
Recently, numerous studies have demonstrated that proinflammatory cytokines, such as IL-1, IL-6, IL-18, TNF-α, and interferon-gamma (INF-γ), are involved in the pathogenesis of AOSD. Regarding the cytokine cascade in AOSD, IL-18 promotes TNF-α and IL-1 production via the nuclear factor-κB pathway and induces INF-γ production by Th1 lymphocytes. TNF-α also induces IL-1, which stimulates the production of IL-6. Based on these facts, IL-18, a macrophage-derived cytokine, is thought to be an upstream cytokine in the pathogenesis of AOSD [3]; on the other hand, IL-6 is thought to be a downstream cytokine.
Recent studies have revealed that higher IL-18 levels are detected in patients with active AOSD, which correlate with disease activity and inflammatory laboratory features. Kawaguchi et al. [4] reported significantly higher IL-18 levels in patients with steroid-resistant AOSD than in those with steroid-sensitive AOSD. The authors suggested that steroid monotherapy was insufficient and that additional immunosuppressive agents should be considered for the treatment of AOSD cases with high IL-18 levels. The present case was considered severely active because of the high ferritin and IL-18 levels; therefore, we administered methyl-PSL pulse therapy followed by PSL and Cy-A. Although this treatment was effective to a certain extent, it failed to completely suppress the disease activity. After using Tac as an alternative to Cy-A, the fever and CRP levels improved completely with gradual improvement in ferritin and IL-18 levels. Murakami et al. [1] reported a case of refractory AOSD with high IL-18 levels who had been treated unsuccessfully with steroid, MTX, Cy-A, infliximab and etanercept, and was subsequently treated successfully using Tac in combination with steroid. The authors reported improvement in the patient's clinical features and laboratory findings including IL-18, TNF-α, and IL-6 levels [1]. Based on these facts, it was suggested that Tac might block the inflammatory cascade of AOSD at an upstream position. Indeed, calcineurin antagonists such as Tac and Cy-A inhibited IL-18 production from lipopolysaccharide-stimulated peripheral blood mononuclear cells in a concentration-dependent manner in vitro [5]. Moreover, Tac elicited a more efficient decrease in IL-18 production than Cy-A at the same molar concentrations [5]. These findings may explain the superiority of Tac to Cy-A for AOSD treatment.
To our knowledge, only two reports have described the successful outcome in two refractory AOSD cases (including the present case) treated with Tac. Although these cases suggest that Tac may be a treatment option for refractory AOSD, further research is required to substantiate the effectiveness of Tac treatment before it can be recommended on a routine basis.
Notes
No potential conflict of interest relevant to this article was reported.