Current evidence of effects of Helicobacter pylori eradication on prevention of gastric cancer
Article information
Abstract
Gastric cancer is the second most common cause of cancer death worldwide and is usually detected at a late stage, except in Korea and Japan where early screening is in effect. Results from animal and epidemiological studies suggest that Helicobacter pylori infection, and subsequent gastritis, promote development of gastric cancer in the infected mucosa. Relatively effective treatment regimens are available to treat H. pylori infection, and in general, mass eradication of the organism is not currently recommended as a gastric cancer prevention strategy. However, regional guidelines vary regarding the indications and recommendations for H. pylori treatment for gastric cancer prevention. In this review, we discuss the results from intervention studies, provide insight regarding current guideline recommendations, and discuss future study directions.
INTRODUCTION
Infection is a major etiology of human cancer and accounts for 2 million cases per year worldwide, equating to a population attributable fraction of 16.1% [1]. Prevention and treatment of infectious organisms, including viruses, bacteria, and parasites, have shown a significant beneficial effect on cancer prevention. After the introduction of Helicobacter pylori to the scientific world as a pathogen for gastritis and peptic ulcer, it was soon suspected as an etiology for gastric cancer (GC). Less than a decade later, two nested case-control studies were published which suggested that infection with H. pylori is strongly associated with an increased risk of gastric carcinoma [2,3]. Such suspicions became much stronger such that the World Health Organization and the International Agency for Research on Cancer declared the organism a group I carcinogen [4]. This declaration was made before any definite animal studies were performed to fulfill Koch's postulates. Thereafter, several animal studies were published in the late-1990s that confirmed gastric carcinogenesis by the organism [5,6]. Meta-analyses that included additional epidemiological studies confirmed the association between the organism and GC [7]. Just after the beginning of the new millennium, a prospective observational study showed that 2.9% of patients with H. pylori infection developed GC in contrast to 0% of H. pylori-negative patients [8].
Although vaccination can prevent cancer-associated viral infectious agents, there has been little success in developing a vaccination for H. pylori infection. Instead, eradication therapy using combinations of antibiotics and proton pump inhibitors has shown relatively high success rates for the organism. Although H. pylori is a treatable infectious organism, the effectiveness, feasibility, and safety of eradication of H. pylori infection to prevent GC in the general population is not yet clear [1]. In this review, current evidence of GC prevention will be discussed for consideration in future recommendations and trials.
CURRENT RECOMMENDATIONS FOR H. PYLORI ERADICATION ON THE PREVENTION OF GC
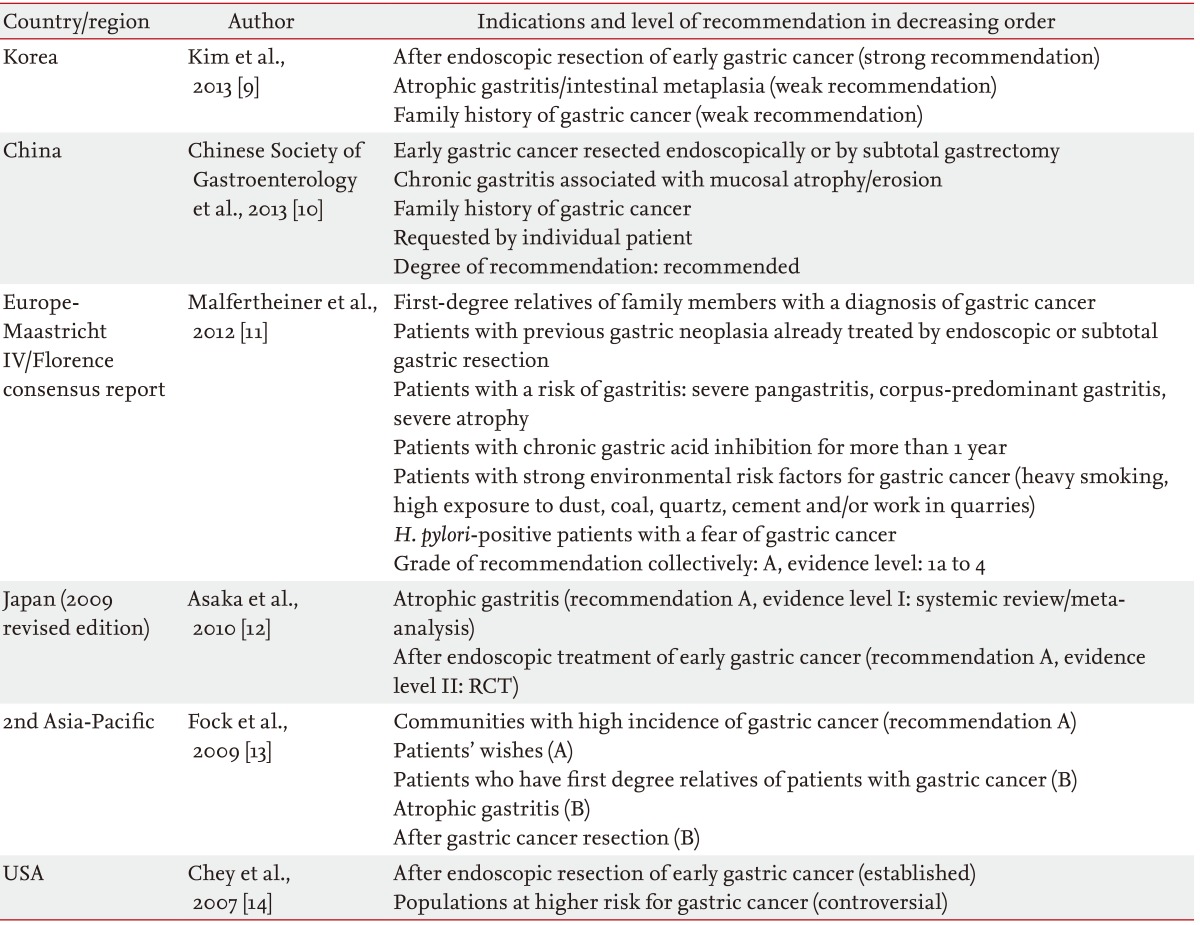
Consensus reports about H. pylori eradication treatments have been published from many geographic regions (Table 1) [9-14]. Consistent indications among these guidelines with high levels of evidence are peptic ulcer diseases and gastric mucosa-associated lymphoid tissue lymphoma (low-grade). Each guideline recommends H. pylori treatment in specific conditions as a preventive tool for GC according to the available evidence [15]. The most consistent recommendation is after endoscopic resection of GC. This indication is included even in Western countries where endoscopic resection for early GC is not yet popular [11]. Other common indications for GC prevention are family members of GC patients, gastric atrophy, and those who desire the regimen. Currently, the only indication with a high level of direct evidence of prevention is after endoscopic resection of early gastric cancer (EGC) [16]. Other conditions do not have any direct evidence that such an approach leads to a decrease in GC incidence. Gastric atrophy can improve after eradication, which was confirmed in many studies [17], but a decrease in GC incidence after eradication in this group of patients has not been proven yet.
H. PYLORI TREATMENT IN THE GENERAL POPULATION
Evidence from randomized controlled study of primary outcome
Although GC is a major problem in Eastern Asian countries including Korea, Japan, and China, it is difficult to conduct a well-designed study to evaluate the preventive effects of H. pylori eradication therapy, because such a study will take a long time and need a large number of participants [18]. The only randomized controlled trial (RCT) that evaluated GC incidence as a primary outcome was performed in a high-risk region of China [19]. This study was a prospective placebo-controlled primary prevention study that enrolled 1,630 healthy H. pylori-infected subjects. During a follow-up period of 7.5 years, seven cases were found in the H. pylori eradication treatment group and 11 cases were found in the placebo group. Reduction of GC was not observed overall (p = 0.33). However, in a subgroup analysis of patients without precancerous lesions, GC did not develop in the H. pylori eradication treatment group compared with six cases of GC in the placebo group (p = 0.02).
The study concluded that incidence of GC development in the general population level was similar between subjects receiving H. pylori treatment and those with placebo medication. The study, however, suggested the possible protective role of H. pylori eradication in participants without precancerous lesions, including gastric atrophy, intestinal metaplasia, or dysplasia. This finding may be a very important issue that will guide future directions in the implementation of H. pylori eradication strategies to prevent GC. However, this was a result from an ad hoc analysis that should be confirmed.
One of the major limitations of the study was that the sample size estimation and follow-up duration were too optimistic [20]. The study design anticipated 3% of GC development in H. pylori carriers (placebo group) compared to 0.99% in the eradicated group during the 7-year follow-up period. Thus, 11 GC patients (1.35%) in the treatment group were far fewer than expected. This may be due to the relatively young age of the participants, which was just over 42 years of age. Even after a 7.5-year follow-up period, the mean age of the participants should be about 50 years. When we consider that GC, especially intestinal types of GC, is a disease of old age, the age of the participants was too young to meet the assumption of the initial sample size calculation.
Evidence from randomized controlled of secondary outcome
Another study was performed in China that evaluated GC development as a secondary outcome measure. The Shandong Intervention Trial, which began in 1995 in Linqu County of Shandong Province, evaluated whether chemopreventive interventions could reduce the development of gastric precancerous lesions [21,22].
A total of 3,365 subjects were randomized in a factorial design into one of three intervention or placebo groups or placebo group: amoxicillin and omeprazole for 2 weeks as an H. pylori treatment; vitamin C, vitamin E, and selenium for 7.3 years as a vitamin supplement; and garlic extract and steam-distilled garlic oil for 7.3 years as a garlic supplement [21]. Although GC developed in fewer individuals in the H. pylori treatment group (19/1,130, 1.7%) than in the placebo group (27/1,128, 2.4%), it was not statistically significant (adjusted p = 0.14).
The results of an extended follow-up of 15 years were recently reported [22]. The subjects of the original study were followed for 14.7 years after an antibiotic treatment was given, and for 7.3 years after the vitamin and garlic supplement treatments ended. GC incidence was 3.0% in the treatment group and 4.6% in the placebo group (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.38 to 0.96; p = 0.032). Deaths from GC occurred among 1.5% of subjects in the treatment group and among 2.1% in the placebo group (hazard ratio [HR], 0.67; 95% CI, 0.36 to 1.28). Thus, the study concluded that the H. pylori eradication treatment with amoxicillin and omeprazole significantly decreased GC incidence by 39% and mortality by 33% for 15 years after treatment.
The major caveat of the study is that H. pylori eradication therapy involved a 2-week amoxicillin and omeprazole treatment for those who were seropositive for H. pylori. The amoxicillin and omeprazole dual therapy is considered suboptimal for H. pylori eradication therapy, with an eradication rate of 65% in pooled analysis [23]. Moreover, seropositivity cannot be directly translated into current infection because of frequent false-positive and -negative results.
In this study, 13C-urea breath tests showed that H. pylori treatment eradicated H. pylori infections in 73% of individuals at the initial evaluation, and only 46% of those who received H. pylori eradication treatment remained negative after 7 years [21]. Thus, in the treatment group, one-fourth of the participants did not respond to the eradication initially and another one-fourth became reinfected during the follow-up. Although frequent treatment failures and high reinfection rates compromise the results, these limitations drive outcomes in favor of accepting the null hypothesis. Thus, this study may provide major support for H. pylori treatment strategies for GC prevention.
H. PYLORI TREATMENT AFTER ENDOSCOPIC RESECTION OF EGC
Results of initial nonrandomized study
Studies trying to evaluate H. pylori eradication effects on GC prevention in the general population may not reach the primary outcome end point, which is GC incidence, if the sample size is not large enough, or the follow-up period is not long enough [18,24]. One of the approaches that can overcome these obstacles is to study a high-risk population. Risk of GC is highest in GC patients who had their EGC treated by endoscopic resection that, as a result, preserved most of their stomach mucosa, which can harbor metachronous cancer. Metachronous GC rates are reported to be about 3% to 4% per year [16,25,26].
Uemura et al. [27] first reported that H. pylori eradication could reduce the subsequent development of metachronous GC after endoscopic resection of EGC. One hundred thirty-two H. pylori serology-positive patients who underwent endoscopic resection were nonrandomly assigned to the H. pylori-treatment group (65 patients) or the no treatment group (67 patients) according to the patients' preference. Regular endoscopic follow-up for up to 48 months found no metachronous cancer in H. pylori-treated patients compared to six cases (9%) in the no treatment group (0.011 by log-rank test). Although the study had the limitations of a nonrandomized study, the present guidelines accepted these results as the basis of an H. pylori eradication therapy after endoscopic resection [28].
Evidence from randomized control studies
A decade after the first publication to support H. pylori eradication after endoscopic resection, another study was published by Japanese researchers with a prospective RCT design [16]. The study was a multicenter, open-label, randomized trial, which recruited 544 patients with EGC treated by endoscopic resection who were evenly randomized to either an eradication regimen (n = 272) or control (n = 272). Metachronous GC had developed in nine patients in the eradication group and 24 in the control group. In the intention to treat analysis, the OR for metachronous GC was 0.353 (95% CI, 0.161 to 0.775; p = 0.009). The trial concluded that eradication of H. pylori after endoscopic resection of EGC should be provided as a prophylactic measure for metachronous cancer prevention [16].
This study has been acknowledged in many guidelines as the sole, well-designed study on this topic [10,11]. The major limitation of the study was an open-labeled design, and the patients were not evaluated blindly during the follow-up endoscopy. Another limitation was that the study included many synchronous cancers, which were detected within 1 year of randomization. According to the Kaplan-Meier plot provided, most of the differences can be explained by those cancers detected within 1 year. The short duration of follow-up is another problem. If H. pylori eradication slows GC growth, metachronous cancer detection might require a longer follow-up duration than 3 years. Long-term results of this RCT were presented in Digestive Disease Week (DDW) 2012, and the data suggested that H. pylori eradication prevented the development of GC after endoscopic resection during a long-term follow-up [29]. Metachronous GC had developed in 22 patients in the eradication group and in 43 in the control group (HR, 0.497; p = 0.008) during the mean 5-year follow-up period.
A Korean RCT that evaluated the effects of H. pylori eradication after endoscopic resection for 664 patients (EGC, n = 408; and adenoma, n = 256) were reported in DDW 2012 [30]. This study has a similar design to a prospective, randomized, and open-label trial. During the median 30 months, new GC had developed in 10 patients in the eradication group and in 15 in the control group (p = 0.85). The cumulative incidence of GC was not significantly different between the treatment and control groups (p = 0.32 by log-rank test), nor between patients with positive and negative final H. pylori status (p = 0.32). The study concluded that prophylactic eradication of H. pylori after endoscopic resection of the tumor was found to exert no significant impact on the subsequent development of metachronous GC.
Results from retrospective studies
Two recently published retrospective studies from Japan raised the follow-up duration problem [25,31]. The first study retrospectively enrolled 268 H. pylori-positive patients who underwent endoscopic resection [31]. The rate of metachronous GC was compared between 177 H. pylori-treated patients and 91 patients with persistent infection. If the follow-up period was limited to 5 years, the incidence rate in the eradicated group was lower than that observed in the persistent group (p = 0.007). However, metachronous GC developed in 13 patients (14.3%) in the persistent group and in 15 patients (8.5%) in the eradicated group (p = 0.262, log-rank test) during the overall follow-up period, ranging from 1.1 to 11.1 years (median, 3.0). Thus, the study concluded that H. pylori eradication does not signif icantly prevent metachronous GC.
Another Japanese study retrospectively evaluated multiple cancers, including synchronous and metachronous cancer after endoscopic resection [25]. The cumulative incidence of metachronous cancer increased linearly, and the mean annual incidence rate was 3.5%, resulting in an overall incidence of 16% in 5 years. The incidence of metachronous cancer that developed 1 year after endoscopic resection in patients who had successful H. pylori eradication (n = 263) was not significantly decreased compared with those who did not receive eradication or for whom eradication failed (n = 105) [25]. These two recent retrospective studies raised a question about the effectiveness H. pylori treatment after endoscopic resection for EGC. Those patients should have a severe premalignant condition in their stomachs, and they should have some risk for GC development, such as an atrophic change or genetic predisposition [32].
Korean retrospective study results are disparate. A study (n = 176) that evaluated the risk factors for metachronous GC after endoscopic resection showed that H. pylori status had no significant effect [33]. However, a large-scale retrospective study (n = 1,487) suggested that H. pylori eradication reduces incidence of metachronous GC in patients with a history of gastric adenoma or EGC. In that study, recurrence rates (17.0% vs. 6.0%, p < 0.01; HR, 2.8) and recurrence-free survival differed significantly between the noneradication and eradication groups [34].
Our recent retrospectively investigated the effect of H. pylori status on the incidence of metachronous GC after endoscopic resection [35]. During the follow-up period of median 4.1 years, metachronous GC developed in 16 patients (16/107, 15%) in the H. pylori-positive group and in 15 patients (15/267, 5.6%) in the H. pylori-negative group. The cumulative incidence of metachronous GC was significantly lower in the H. pylori-negative than in the H. pylori-positive groups (p = 0.003 by log-rank test). In a multivariate Cox proportional analysis, H. pylori-positive status (HR, 2.52; 95% CI, 1.24 to 5.14; p = 0.011) was associated with the development of metachronous GC.
Taken together, effects of H. pylori eradication to prevent metachronous GC should be evaluated in additional well designed long-term studies. Such studies will provide powerful evidence that H. pylori eradication can eventually decrease GC development in high-risk persons not only with EGC but also with advanced atrophy and/or intestinal metaplasia.
IN PATIENTS WITH SUBTOTAL GASTRECTOMY
Many guidelines recommend H. pylori treatment after GC resection. However, the terms are sometimes vague and not-well defined as to whether H. pylori eradication is recommended after surgery and whether the guidelines pertain to cases of advanced gastric cancer (AGC).
Guidelines from Korea and Japan recommend H. pylori eradication, specifically after endoscopic resection of EGC [9,12]. Chinese guidelines appear to expand the indication to EGC treated with subtotal gastrectomy [10]. Asia-Pacific guidelines recommended resection for all GC, which encompasses both EGC and AGC treated with subtotal gastrectomy [13]. European guidelines also recommend H. pylori eradication in patients with previous gastric neoplasia already treated with endoscopic or subtotal gastric resection, which seems to broaden the indication to include adenoma or dysplasia under the terminology of neoplasia as well as all GC treated with endoscopic resection or subtotal gastrectomy [11].
At present, there have been no studies that primarily evaluated whether H. pylori eradication can decrease GC in the remaining stomach. Subtotal gastrectomy for distal GC removes about two-thirds of the gastric mucosa, which is the most probable region to develop metachronous cancer. Moreover, the physiology of the remnant stomach after subtotal gastrectomy differs from that of the normal stomach. Bile reflux can suppress or even clear H. pylori from the remnant stomach and may act as a carcinogen for later GC development [36,37].
In our RCT of patients (n = 190) who underwent subtotal gastrectomy, metachronous GC developed in four patients, including three patients in the H. pylori-treatment group and one patient in the placebo group during a median 60 months of follow-up [38]. When final H. pylori status was evaluated, two of the four patients had persistent H. pylori infection because one patient in the treatment group had failed the treatment. Therefore, there was no difference in the development of metachronous GC according to the treatment allocation or final H. pylori status. This observation should be evaluated in further studies.
IN PATIENTS WITH ATROPHY OR INTESTINAL METAPLASIA
The Correa hypothesis states that H. pylori-induced gastritis progresses to gastric atrophy and intestinal metaplasia, eventually leading to GC development. Differences in GC incidence can be partly explained if a difference in the prevalence of severe gastritis was noted. There have been comparative studies that showed that H. pylori-infected Japanese patients had much higher prevalence of premalignant conditions than the population from low-risk regions including United Kingdom and Asian countries such as Indonesian or Bangladeshis patients [39-41]. These differences might be associated with host genetic differences that determine levels of proinflammatory cytokine production such as interleukin (IL)-1β, tumor necrosis factor-α, or anti-inflammatory cytokines, such as IL-10 [42,43]. Bacterial factors such as cag-associated pathogenicity islands that lead to production of the cagA protein are also associated with an increased severity of gastritis and subsequent GC risk [44,45]. In Korea, bacterial virulence factors and host genetic factors (both pro- and anti-inflammatory cytokines) are important risk factor for atrophic gastritis and intestinal metaplasia [46].
Recently published studies raise a question about long-term GC preventive effects by H. pylori eradication if histological changes have already become severe. A Western case study of two patients who suffered benign gastric ulcer before H. pylori treatment developed GC 4 and 14 years after H. pylori eradication, respectively [47]. An RCT performed by Wong et al. [19] showed no overall beneficial effect in participants but suggested that H. pylori eradication was beneficial in patients without premalignant conditions. H. pylori eradication did not have any effects in patients with advanced premalignant gastric conditions.
Atrophic gastritis and intestinal metaplasia increase GC risk significantly, and the reversibility of these two histological premalignant conditions has been controversial. A meta-analysis showed that H. pylori eradication can improve atrophy but not intestinal metaplasia [17]. GC suitable for endoscopic treatment is intestinal type by Lauren's classification and usually has atrophy and intestinal metaplasia at background mucosa. In this context, preventive effect of H. pylori treatment of metachronous GC after endoscopic resection has important implication that H. pylori eradication may be helpful even in far advanced histological changes [16].
IN PATIENTS WITH FAMILY HISTORY OF GC
A positive family history of GC is a well-established risk factor [48]. The possible contribution of family history seems multifactorial. The first-degree relatives of GC patients share factors that contribute to the risk of GC, such as genetic factors, including genetic polymorphisms, and environmental factors, especially during childhood [49].
A meta-analysis of H. pylori infection and gastric histology in family members showed that first-degree relatives had a significantly higher prevalence of H. pylori infection. They also have a higher prevalence of glandular atrophy and intestinal metaplasia than controls [50]. According to the meta-analysis, the magnitude of pooled OR with 95% CI was 1.925 (1.419 to 2.611) for H. pylori infection, 2.200 (1.266 to 3.824) for atrophy, and 1.982 (1.363 to 2.881) for intestinal metaplasia. A recent Korean study showed increased infection rates of H. pylori and higher grade of intestinal metaplasia in the corpus gastric mucosa in young family members of early onset GC patients diagnosed before the age of 40 years [51]. In a Western population, the first-degree relatives of GC patients were also confirmed to have a high prevalence of H. pylori infection, atrophy, and intestinal metaplasia even at young age [52].
Currently available guidelines recommend H. pylori eradication in relatives of GC patients. However, there seems to be no direct evidence that eradication policies can reduce GC incidence in this high-risk population.
TRIALS OF NONRANDOMIZED DESIGN OR SECONDARY OUTCOME MEASURES
Nonrandomized clinical trials that evaluated GC incidence in eradicated and noneradicated subjects who underwent endoscopic follow-up were reported from Japan (Table 2) [53-57]. The results among these studies are disparate, and three studies showed preventive effects of H. pylori eradication on GC development [55-57]. Those studies enrolled peptic ulcer patients and showed significant risk reduction in a relatively short follow-up duration of just more than 3 years. Benign gastric ulcer or duodenal ulcer patients have different clinical characteristics that affect GC incidence [58]. Gastric ulcer is associated with corpus predominant gastritis or pangastritis, and increased risk of GC, whereas duodenal ulcer is associated with antral-predominant gastritis and GC risk is reduced [8,59]. A GC prevention study using patients with peptic ulcer, including both gastric and duodenal ulcers, may yield inconsistent or null results according to the population composition.
Two large-sized studies that enrolled more than 4,000 participants who were followed up for more than 5 years showed no significant results [53,54]. One of the studies enrolled peptic ulcer patients, but it was unclear whether nonrandomized studies collectively support any evidence of H. pylori treatment as a preventive measure in healthy people.
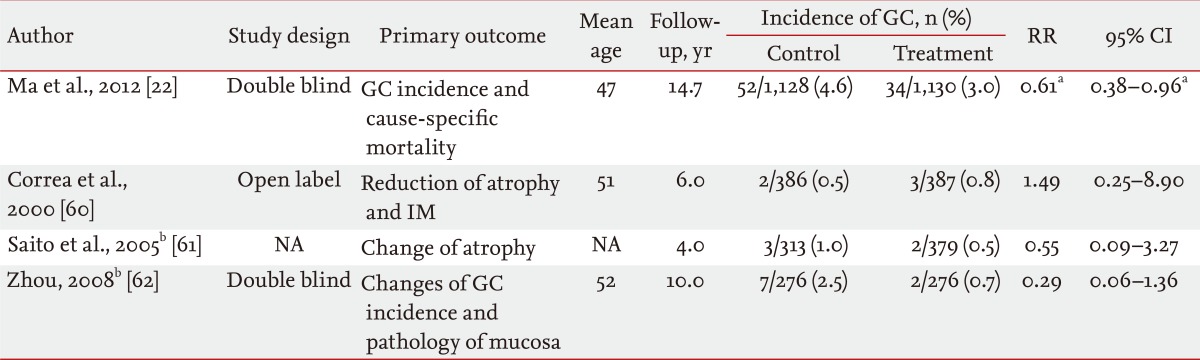
Three randomized studies that evaluated effects of H. pylori eradication on cancer as a secondary outcome measure showed nonsignificant results (Table 3) [60-62]. Two of the studies were reported only in abstract form and full papers are not yet available; as such, the calculated relative risks of both studies were not significant [58]. Only one published study of long-term follow-up showed a significant risk reduction as discussed above [22].
PROGNOSIS IN CASES OF H. PYLORI-NEGATIVE GC
While H. pylori infection is the most important risk factor for GC development, there have been suggestions that H. pylori infection is also a prognostic factor. A German study assessed the H. pylori status of 166 GC patients with R0 resection and showed that H. pylori-negative status yielded a significantly shorter OS of 19.2 months compared to 61.9 months for H. pylori-positive patients [63]. Another study from Italy evaluated 297 GC patients and showed a lower 5-year survival rate after R0 resection in H. pylori-negative patients (24% vs. 57%; p < 0.001) compared to that of H. pylori-positive patients, a finding that was independent of other wellknown risk factors [64]. A Korean study also showed that for 61 patients with stage III or IV GC, the disease-free survival of patients negative for H. pylori infection was significantly shorter than for patients with a positive H. pylori status (23.6 months vs. 31.5 months; p = 0.019) [65].
Recently, a meta-analysis was published that evaluated the association between H. pylori infection status and GC prognosis [66]. Although there have been no RCTs that evaluated the association of H. pylori status and prognosis of already developed GC, a meta-analysis was applied to identify or confirm prognostic factors. Pooled analysis of 12 studies, including a total of 2,454 patients with GC, was performed. The result showed that the pooled HR was 0.71 (95% CI, 0.57 to 0.87; p = 0.001) for OS and 0.60 (95% CI, 0.30 to 1.18; p = 0.139) for DFS in GC patients. An observed HR of less than one implied a better OS for patients with H. pylori-positive status compared with the reference group with H. pylori-negative status. A protective role of H. pylori infection on GC prognosis was demonstrated among subgroups stratified by ethnicity (Caucasian), H. pylori evaluation method (serology and histological evaluation), and quality assessment (high quality). Interestingly, the association was not found in Asians, where GC incidence is highest. The major limitations of the meta-analysis are the significant heterogeneity between individual studies, especially for the diagnostic method of H. pylori status and the retrospective design of most of the included studies.
The finding that H. pylori infection improves outcome for GC patients is intriguing, because the organism is the major risk factor for malignancy. A plausible explanation for this apparent paradoxical observation is that H. pylori-induced inf lammation may induce a cellular immune response and subsequently enhance the antitumor activity of immune system [66]. Another possible explanation is the involvement of microsatellite instability. Microsatellite alterations are closely related to H. pylori infection and subsequently render a better postoperative survival in GC cases with H. pylori infection compared to uninfected cases of GC [66].
The proportion of GC cases not associated with H. pylori is approximately 5% or less in Korea [67,68] and about 10% or less than 1% in Japan, according to the exclusion level of atrophy [69,70]. Most H. pylori-negative GCs are associated with a past H. pylori infection based on the underlying gastric mucosal histology, showing atrophy or intestinal metaplasia. To elucidate whether H. pylori status really affects GC prognosis and whether current recommendation of H. pylori treatment after surgery does not worsen the outcome, prospective RCTs with rigorous H. pylori status evaluation using combined diagnostic method and with comparable allocation groups are needed in the future.
PERSPECTIVES TO PREVENT GC IN HIGH-RISK AREAS
Strategies to prevent GC development by H. pylori treatment are fascinating and mechanistically plausible approaches. However, the current evidence does not provide definite backgrounds that H. pylori eradication is beneficial overall. There have been suggestions that H. pylori eradication is associated with increased prevalence of asthma, obesity, reflux esophagitis, and gastric cardia cancer in GC low risk regions [71-73]. Antibiotics use may raise concerns not only for bacterial resistance but also for the permanent changes of our protective gut microflora which could have more serious consequences [74]. Moreover, all cause mortality was not associated with H. pylori seropositivity in two studies performed in the United States [75,76]. Thus, H. pylori eradiation may not reduce all-cause mortality significantly despite its preventive effects on GC. This finding appears to be more relevant in low-risk areas, and whether this finding can be extrapolated into high-risk areas has yet to be determined.
According to Asia-Pacific guidelines, H. pylori eradiation is recommended in high-risk regions for GC [13]. Recently, this strategy was approved in Japan by the government to reimburse gastritis patients for H. pylori treatment by the National Health Insurance system. Japanese researchers eagerly promoted this option to decrease GC incidence [77]. Different measures are suggested for people aged below 20 years and people aged 50 years or older [78]. In persons aged below 20 years, bacterial eradication after test is recommended to achieve GC prevention. People aged 50 years or older are recommended to eradicate H. pylori and to have endoscopic surveillance according to the presence and severity of gastritis.
Korea has the world's highest incidence of GC. The Korean government and health insurance system now provide mass screening using endoscopy or an upper gastrointestinal series according to the preference of the participants [79]. This 2-year interval screening program is unique in the world and may provide a very effective means for secondary prevention, because most GCs detected during this screening are EGCs [80]. Under this screening policy for GC in Korea, H. pylori eradication will not be readily reimbursed without confirmative evidence of GC prevention. However, as baby boomers are getting older and entering the GC-prone age group, the budget for GC screening will expand substantially. Although upper endoscopy costs are very low and appear to be cost effective [81], screening endoscopy without risk stratification will cause significant burden in the future.
CONCLUSIONS
The association of H. pylori infection with GC has been more strongly recognized during the past two decades. However, no definite, well-designed study has shown the prevention of GC by H. pylori eradication in the general population that can support mass eradication policies. Considering historical experience and plausible carcinogenesis mechanisms, young individuals in high-risk regions may be the best candidates for eradication. Although the point of no return in terms of gastric mucosal changes renders doubt on the strategy of H. pylori eradication in aged persons, further study is needed. Globally, we should address approaches to reduce GC mortality as we are expecting a marked increase in the aged population in high-risk regions.
Acknowledgments
This work was supported by grant from the National Cancer Center, Korea (1310280).
Notes
No potential conflict of interest relevant to this article is reported.