Analysis of 18F-fluorodeoxyglucose positron emission tomography findings in patients with pituitary lesions
Article information
Abstract
Background/Aims
Although magnetic resonance imaging (MRI) is a good visual modality for the evaluation of pituitary lesions, it has limited value in the diagnosis of mixed nodules and some cystic lesions. We evaluated the usefulness of 18F-fluorodeoxyglucose positron emission tomography (FDG PET) for patients with pituitary lesions.
Methods
18F-FDG PET and MRI were performed simultaneously in 32 consecutive patients with pituitary lesions. The relationships between FDG uptake patterns in PET and MRI findings were analyzed.
Results
Of 24 patients with piuitary adenomas, 19 (79.2%) showed increased uptake of 18F-FDG in the pituitary gland on PET scans. All patients with pituitary macroadenomas showed increased 18F-FDG uptake on PET scans. Meanwhile, only five (50%) of the 10 patients with pituitary microadenomas showed positive PET scans. Interestingly, of two patients with no abnormal MRI findings, one showed increased 18F-FDG uptake on PET. For positive 18F-FDG uptake, maximum standardized uptake values (SUVmax) > 2.4 had 94.7% sensitivity and 100% specificity. In addition, SUVmax increased in proportion to the size of pituitary adenomas. Most cystic lesions did not show 18F-FDG uptake on PET scans.
Conclusions
About 80% of pituitary adenomas showed positivity on PET scans, and SUVmax was related to the size of the adenomas. PET may be used as an ancillary tool for detection and differentiation of pituitary lesions.
INTRODUCTION
In 1899, X-ray provided the first visual documentation of an enlarged sella turcica in a patient with acromegaly [1]. In the 1970s and 1980s, computed tomography (CT) and magnetic resonance imaging (MRI) were introduced into clinical practice. These imaging technologies revolutionized the diagnosis and management of patients with pituitary diseases [1]. MRI, with improved resolution, has since the 1990s been the first choice for diagnosing pituitary lesions [2]. MRI can display surrounding structures as well as pituitary lesions [3]. Nevertheless, differentiating some cystic lesions from mass-related changes, such as cystic changes or hemorrhage, by MRI is sometimes problematic [4].
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) is a valuable method for the diagnosis and staging of malignancies as well as monitoring their therapeutic effectiveness. PET is also helpful for some diagnoses that are difficult to obtain with MRI. However, PET has not been frequently used for the diagnosis of pituitary tumors. There are limited data on the diagnostic value of PET for pituitary tumors.
In this regard, we experienced one interesting case involving Rathke's cleft cysts (RCC) (case 27). A 59-year-old woman was referred to the hospital due to symptoms of panhypopituitarism and visual disturbances. Because brain MRI revealed a focal enhanced mass extending over the pituitary gland, optic chiasm, and bilateral optic tracts (Fig. 1A), she was first diagnosed with optic glioma. Her symptoms spontaneously improved, and she was observed for 3 months. A subsequent MRI showed improvement in the lesions surrounding the optic tract, whereas a cystic lesion in the pituitary fossa had expanded. A PET scan was performed, and it showed no uptake of 18F-FDG in the pituitary fossa (Fig. 1B). This suggested that it was more likely to be a cystic lesion such as RCC. She underwent trans-sphenoidal surgery for confirmation and was finally diagnosed with RCC.

Magnetic resonance imaging (MRI) findings and 18F-fluorodeoxyglucose positron emission tomography (FDG PET) imaging in a 58-year-old woman with Rathke's cleft cyst (case 27). (A) T1-weighted MRI with gadolinium contrast. Coronal (upper panel) and axial (lower panel) views demonstrate an enhanced mass (white arrows) covering the pituitary gland and optic tracts. (B) Sagittal view of the brain on PET imaging showed no abnormal 18F-FDG uptake in the sella turcica (black arrow).
Thus, we investigated the usefulness of 18F-FDG PET for the diagnosis and evaluation of various pituitary lesions.
METHODS
Patients
From January 2005 to August 2010, we performed PET and MRI simultaneously in 46 consecutive patients who had been investigated for pituitary lesions according to presenting symptoms, signs, laboratory data, or other imaging studies. We excluded other brain lesions, including brain parenchymal tumors other than pituitary tumors or metastatic brain tumors. A total of 32 patients were diagnosed with pituitary lesions. We obtained informed consent from all patients, and this study was conducted with the approval of the Ethics Committee at Severance Hospital, Yonsei University College of Medicine.
PET scanning
All patients fasted for more than 6 hours prior to the test. Sixty minutes after intravenous injection of 7 to 9 mCi of 18F-FDG, images were obtained using a GE ADVANCE PET scanner (GE, Milwaukee, WI, USA). Emission scanning continued for 15 minutes (4.25 mm axial spatial resolution, 4.8 mm transaxial spatial resolution). Transmission scans were performed for 8 minutes using triple Ge-68 rod sources to correct for attenuation. Gathered data were reconstructed in a 128 × 128 × 35 matrix with a pixel size of 1.95 × 1.95 × 4.25 mm by means of a filtered back-projection algorithm employing a transaxial 8.5-mm Hanning filter and 8.5-mm axial ramp filter. Two experienced nuclear medicine doctors evaluated the 18F-FDG PET images on a high-resolution computer screen. The standardized uptake value (SUV) was calculated as the concentration of 18F-FDG uptake divided by injected dose/body weight. To avoid a partial volume effect, the maximum SUV (SUVmax) was measured within the region of interest.
MRI
MRI was performed at 1.5 T (Intera Achieva, Philips Medical Systems, Best, Netherlands). T1- and T2-weighted spin-echo images were obtained in the coronal and sagittal planes at 3 mm sections. T1-weighted images were then obtained after intravenous administration of 0.1 mmol/kg of gadolinium gadolinium diethylenetriamine pentaacetic acid.
Statistics
All data are shown as means ± standard deviations. Fisher's exact test and the Mann-Whitney test were applied for comparisons of pituitary lesion characteristics. The Kruskal-Wallis test was used to compare the differences in SUVmax of pituitary cysts, microadenomas, and macroadenomas. The statistics program used for the analysis was the SPSS package for Windows version 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
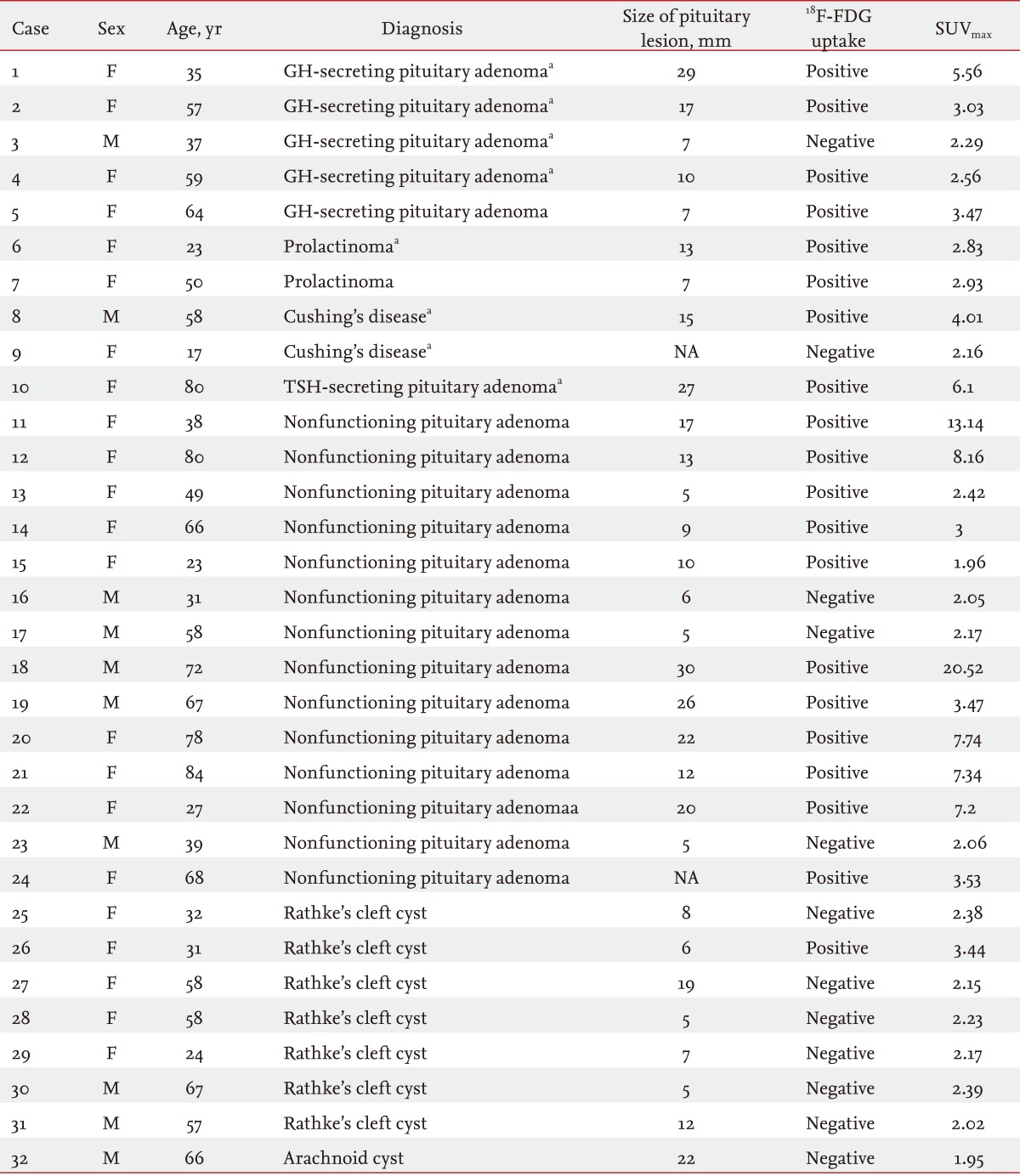
In this study, a total of 32 patients who showed pituitary lesions were included. Females (n = 22) comprised a larger proportion than males (n = 10), and the mean age of the patients was 51.6 years (range, 17 to 80). Twenty-four patients had pituitary adenomas, and eight had cystic lesions. Of the 24 pituitary adenomas, 10 were classified as functioning tumors and 14 as nonfunctioning tumors. There were five patients with growth hormone-secreting adenomas, two with prolactinomas, two with Cushing's disease, and one with a thyroid-stimulating-hormone-secreting adenoma. Among the eight patients with cystic lesions, seven had RCCs, and one had an archnoid cyst (Table 1).
As shown in Table 2, of the 24 patients with pituitary adenomas, 19 (79.2%) showed increased uptake of 18F-FDG on PET scans with a hypermetabolic focus in the pituitary gland. On the other hand, uptake of 18F-FDG on PET was not observed in most cases of cysts (n = 8). One exceptional case of cystic lesions showed suspicious 18F-FDG uptake in the pituitary gland on PET, but the uptake was very low. The total number of pituitary macroadenomas (mean size, 18.6 ± 7.0 mm) was 14, and all (100%) showed positive 18F-FDG uptake on PET scans (Table 2, Fig. 2). The total number of pituitary microadenomas was 10, of which five (50.0%) showed positive 18F-FDG uptake on PET scans. Positive uptake of 18F-FDG was shown in eight of 10 (80.0%) functioning adenomas and 11 of 14 (78.6%) nonfunctioning adenomas (Table 2).
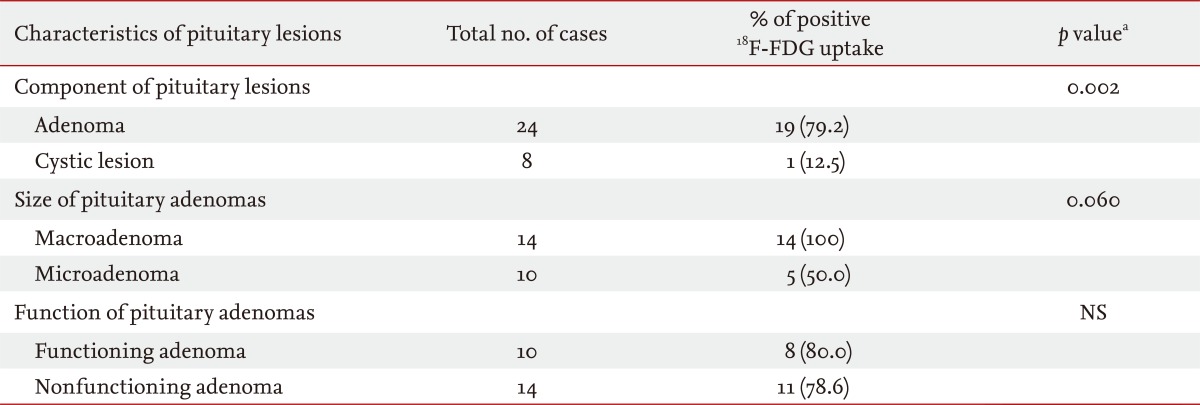
18F-FDG uptake positivity on positron emission tomography according to the characteristics of pituitary lesions
The SUVmax of 18F-FDG on PET scans ranged from 1.9 to 20.5. The mean SUVmax of macroadenomas was significantly higher than that of microadenomas or cystic lesions (6.6 ± 5.1, 2.6 ± 0.5, and 2.3 ± 0.4; p < 0.05, respectively) (Fig. 3). There was a correlation between the size and SUVmax of tumors (r = 0.559, p < 0.01). A > 8.5-mm-maximal diameter of pituitary lesions had 78.9% sensitivity and 72.7% specificity for positive 18F-FDG uptake on PET scans. A SUVmax of > 2.4 had 94.7% sensitivity and 100% specificity for positive 18F-FDG uptake. Although it was not significant, the mean SUVmax of nonfunctioning pituitary adenomas tended to be higher than that of functioning adenomas (mean SUVmax, 7.1 ± 5.5 vs. 3.8 ± 1.3).
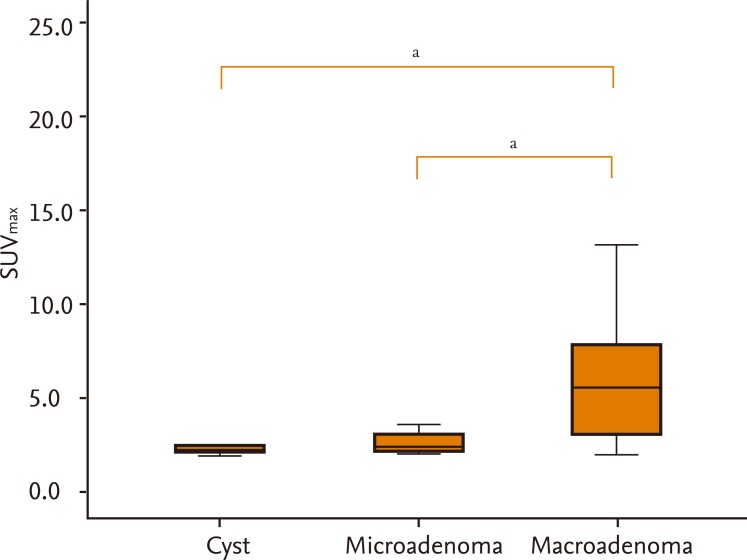
Distribution of maximum standard uptake values (SUVmax) of pituitary lesions (Kruskal-Wallis test). ap < 0.05.
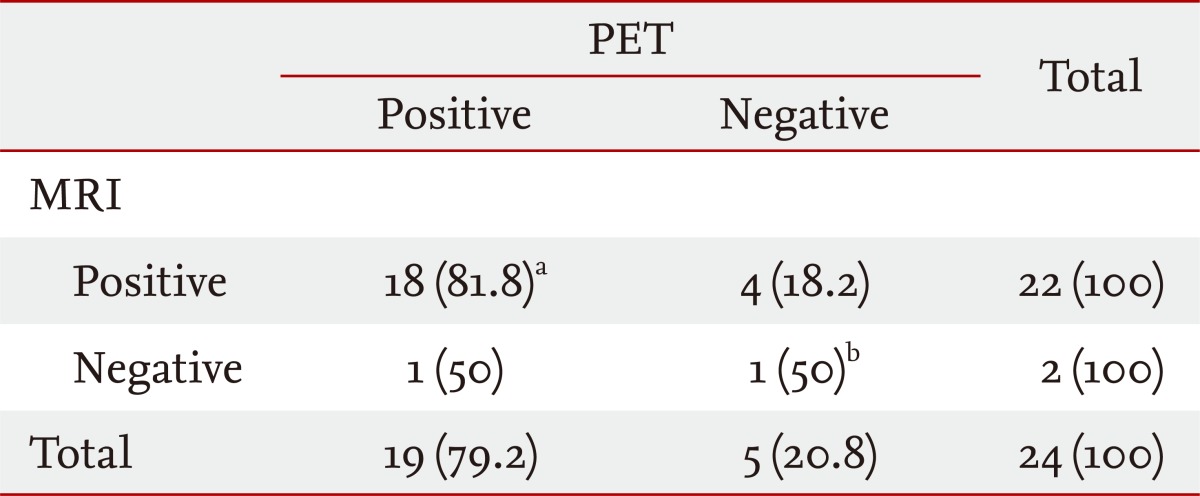
Table 3 shows the PET scan and MRI findings in 24 patients with pituitary adenomas. Of these, 22 (91.6%) showed positive findings on MRI. Of the two who did not show any abnormal findings on MRI, one (case 24) showed positive 18F-FDG uptake on PET. The remaining patient (case 9) showed negative FDG uptake on PET. Although this 17-year-old female patient presented with prominent symptoms of hypercortisolism, neither MRI nor PET showed any abnormal findings. She was diagnosed with Cushing's disease based on inferior petrosal sinus sampling and underwent trans-sphenoidal surgery.
DISCUSSION
MRI is an important method with which to investigate the anatomical structure of pituitary lesions. Nevertheless, differential diagnosis is occasionally difficult for cystic lesions such as cystic or hemorrhagic pituitary adenomas, primary parasellar lesions with presentation in the sellar region, and sometimes RCC [5,6]. Interestingly, in case 27, MRI did not allow for a differential diagnosis between RCC and optic glioma; in case 24, an invisible lesion on MRI was positive on the FDG PET scan. In addition, MRI generally provides little information about the biochemical and functional characteristics of pituitary tumors.
PET emerged as a functional imaging modality in the 1970s for the first time and has since become an important diagnostic technique for many diseases. PET measures the local concentration of tracers, obtains biochemical information such as glucose uptake, and converts them into images. The molecule most commonly used as a radiotracer is 18F-FDG; however, other diverse tracer molecules have been developed and used in various fields. In tumors or metabolically active cells, glycolysis is enhanced, and thus the 18F-FDG uptake rate increases competitively [7,8]. Because the size of the normal pituitary gland is small and its metabolic rate is low, it does not show 18F-FDG uptake on PET scans [9,10]. On the other hand, pituitary adenoma and craniopharyngioma, the cells of which are more metabolically active than normal cells, induce increased 18F-FDG uptake on PET scans. The 18F-FDG uptake rate in pituitary adenoma is 30% higher than that in the whole brain [11].
Recently, several cases of pituitary adenomas were detected incidentally by 18F-FDG PET [12], as in our case 24. One study reported an incidence of pituitary incidentaloma by 18F-FDG PET imaging combined with CT (PET-CT) of 0.073% (30 of 40,967 patients) [13]. This rate is markedly lower than that of incidentalomas by MRI or CT (3.7% to 20%) [14,15]. Several factors may contribute to this difference. First, whole-body PET has a lower spatial resolution than MRI for pituitary lesions. Second, not all pituitary tumors are FDG-avid. Third, there is still no consensus regarding pituitary uptake. 18F-FDG PET as a modality for diagnosis of pituitary lesions has been investigated. Most of these studies involved specified groups, such as patients with Cushing's disease, acromegaly, or other functioning and/or nonfunctioning adenomas. In our study, we investigated the usefulness of PET as an initial diagnostic imaging study in various pituitary lesions. Most cystic lesions did not show 18F-FDG uptake. Only one patient with RCC (case 26) showing heterogeneity on MRI had suspicious uptake of 18F-FDG with a low SUVmax on PET. Earlier studies demonstrated the usefulness of 18F-FDG in patients with pituitary adenomas. De Souza et al. [9] reported that five of 12 patients with positive PET scans showed negative or questionable lesions on MRI and that there were no false-positive cases among all 20 control subjects. In this study, the PET positivity rate in microadenoma was comparable with that of MRI (60% vs. 65%, respectively). PET-CT, rather than PET alone, is now used frequently. One study investigated the diagnostic value of fused 18F-FDG PET-CT in patients with Cushing's disease [16]. They concluded that PET-CT may be an important diagnostic tool for diagnosis of Cushing's disease because PET-CT might identify some Cushing's-positive patients that had a negative MRI [16]. In our study, the overall adenoma positivity on PET was 79.2%. All patients with pituitary macroadenomas showed positive PET scans (100%), whereas positive PET scans were shown only in 50% of microadenomas. SUVmax may facilitate more accurate diagnosis, especially in patients with pituitary microadenomas. Our data showed that a SUVmax of > 2.4 had 94.7% sensitivity and 100% specificity for positive 18F-FDG uptake. However, SUVmax may vary depending on many factors, such as the size of the mass, standardized measurement times, plasma glucose levels, recovery coefficients, and partial volume effects. Thus, further studies of diagnosis using SUVmax with a greater numbers of subjects should be conducted.
Mean SUVmax values were higher in macroadenomas than in microadenomas or cystic lesions (Fig. 3). They also tended to be higher in nonfunctioning adenomas than in functioning adenomas, although there was no difference in the sizes of these adenoma types (13.8 ± 8.4 mm vs. 14.6 ± 8.3 mm, respectively). Similarly, in previous studies it was reported that the 18F-FDG uptake levels of nonfunctional pituitary adenomas were higher than those of functional pituitary adenomas [11,17]. The cause of the higher 18F-FDG uptake by nonfunctional pituitary adenomas has not been elucidated. A possible explanation is that in nonfunctional pituitary adenomas, energy metabolism rates are higher due to incomplete hormone synthesis by the tumor [11]. Second, in nonfunctional pituitary adenomas, inhibition by the final product is inefficient; thus 18F-FDG uptake may be retained [11]. Finally, because 50% of nonfunctional pituitary adenomas show oncocytic changes, higher 18F-FDG uptake may have occurred. In oncocytomas, which overexpress mitochondria, overrepresentation of the hexokinase enzyme leads to increased 18F-FDG uptake [17].
18F-FDG PET may facilitate differentiation of recurred tumors, residual tumors, inflammatory reactions after surgery, and fibrosis [9,18]. After surgery, when the diagnosis of residual or recurred cancer with MRI is unclear due to inflammation or fibrosis, PET may assist their differentiation [11]. 18F-FDG PET also enables prediction of the treatment response and growth potentiality of pituitary adenomas. Bromocriptine decreased the 18F-FDG uptake rates of prolactinomas by up to 20%, and octreotide decreased the 18F-FDG uptake rates in thyroid stimulating hormone- and growth hormone-producing adenomas by up to 53% [11]. Radiation therapy reportedly leads to a 20% to 30% decreased 18F-FDG uptake [11,18]. Therefore, the treatment outcome can be assessed by comparing the PET findings before and after treatment. Because estimation of the functional treatment response, especially in nonfunctioning pituitary adenomas, is problematic, changes in 18F-FDG uptake may in such cases assist determination of treatment responses.
In conclusion, 18F-FDG PET may represent an ancillary tool for detection and differentiation of pituitary lesions in certain circumstances. Further PET studies to determine the appropriate SUVmax threshold, or conjugation of alternative tracer molecules, may facilitate more accurate diagnosis of pituitary lesions. In addition, studies of the change in FDG uptake rates on PET induced by various treatment methods for each pituitary disease will be informative.
KEY MESSAGE
1. In this study, about 80% of pituitary adenoma showed positivity on positron emission tomography (PET) scan and maximum standardized uptake value (SUVmax) was related to the size of adenomas.
2. PET could be useful method for detecting and differentiating various pituitary lesions.
3. Further PET studies determining the right threshold of SUVmax or conjugating various tracer molecules will be helpful.
Notes
No potential conflict of interest relevant to this article is reported.