Adenocarcinoma Arising in a Duplication of the Cecum
Article information
Abstract
Intestinal duplications are rare developmental abnormalities that may occur anywhere in the gastrointestinal tract. The possibility of a malignant change occurring in these duplications is very low. We present a case of adenocarcinoma arising in a duplication of the cecum. A 41-year-old male patient was admitted because of a palpable abdominal mass. Abdominal computed tomography revealed a 6-cm, peripheral wall-enhanced, round, cystic mass in the cecal area. Excision of the mesenteric mass and a right hemicolectomy was performed. Upon histologic examination, the patient was diagnosed with adenocarcinoma arising in a duplication of the cecum.
INTRODUCTION
Intestinal duplications are uncommon congenital abnormalities that may arise anywhere in the gastrointestinal tract [1]. The ileum is the most common area; cecal duplication is very rare [2]. Duplications may present a variety of clinical findings, including acute intestinal obstruction, palpable abdominal mass, vague abdominal pain over prolonged periods, or intermittent gastrointestinal bleeding, nausea, vomiting, abdominal distension, and constipation [2]. Clinical findings are usually related to the location and size of the duplication [2]. Malignancy arising in an intestinal duplication is extremely rare [3]. Here, we present a case of adenocarcinoma arising in a duplication of the cecum.
CASE REPORT
A 41-year-old man presented with a palpable abdominal mass and loose stool, which had developed two months previously. There was no history of disease except for pulmonary tuberculosis, which had completely resolved. Physical examination revealed an approximately 4 × 4 cm, round, non-tender, mobile mass in the right lower quadrant of the abdomen. There was no cervical, axillary, or inguinal lymphadenopathy and no hepatosplenomegaly. The complete blood count, liver function, and kidney function test results were normal. The serum tumor marker, carcinoembryonic antigen (CEA), was within a normal range (1.55 ng/mL; normal range, < 5.0 ng/mL), but cancer antigen 19-9 (CA 19-9) was elevated (82.03 U/mL; normal range, < 37 U/mL). Abdominal computed tomography (CT) revealed a 5 × 4.8 × 6 cm, peripheral wall enhanced, round cystic mass in the cecal area (Fig. 1A). Peripheral calcification and an enhanced solid component were observed in the lower portion (Fig. 1B). No regional lymph node, ascites, or other abnormalities were found. Results of a colonoscopy performed at another hospital several months earlier were normal. During surgery, the cystic mass was observed to be loosely attached to the cecal serosal surface (Fig. 2). It was dissected free and removed intact, then submitted to the department of pathology for frozen section diagnosis; results indicated adenocarcinoma, possibly arising in a cecal duplication cyst. A right hemicolectomy was subsequently performed. Upon gross examination, the spherical cystic mass was tender and firm, and the outer surface was smooth and glistening with a small amount of omental soft tissue attached. Upon opening, the cystic mass revealed a unilocular space containing abundant brown mucinous fluid. The inner surface was smooth and showed multiple brownish spots. The wall was approximately 0.5 cm thick. On serial sectioning, the cut surface revealed a localized grayish white and friable lesion that had infiltrated the adjacent tissue (Fig. 3).

Abdominal computed tomography scan. (A) A 5 × 4.8 × 6 cm, peripheral wall enhanced, cystic mass is noted in the cecal area (arrow). (B) Peripheral calcification and enhanced solid component in the lower portion (arrow).

Bisected cystic mesenteric mass is well-defined and shows a focal area of tumor infiltration into the mesenteric soft tissue (arrow). Inner wall of the cystic mass is relatively smooth. No transition or connection with the intestinal wall is present.
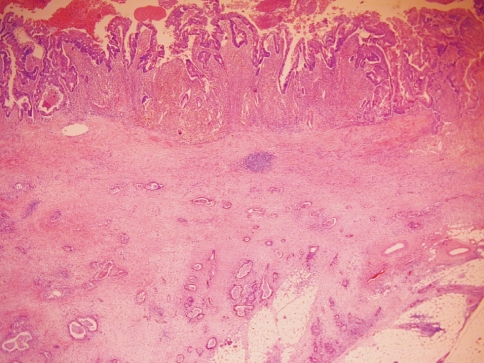
Upon microscopic examination, the cystic mass was found to have a circumferential muscular layer with inner circular and outer longitudinal fibers, similar to those of the intestinal wall (Fig. 4). Despite the multiple sections of the cystic mass, most areas of the internal surface were denuded. No benign glandular epithelium was observed within the wall; instead, it showed the focal area of an atypical glandular lesion composed of an atypical colonic-type mucinous epithelium lining the cyst, which was compatible with in situ adenocarcinoma (Fig. 4, inset). In the mesenteric infiltrative area, there were exuberant infiltrative nests of moderately-differentiated adenocarcinoma exhibiting a solid and tubular growth pattern, which had invaded the pericystic mesenteric soft tissue (Fig. 5). As for the right hemicolectomy specimen, it was grossly unremarkable. The cecum near the cystic mass was clear with no evidence of true connection or adhesion. Neither was there evidence of malignancy, even microscopically. For these reasons, we concluded that this was a moderately-differentiated adenocarcinoma arising in a totally independent cecal duplication cyst (modified Dukes' B2, stage IIA, T3N0M0 as recognized by the American Joint Committee on Cancer). Because lymphatic tumor emboli presented, we regarded the patient as high-risk. We planned adjuvant chemotherapy with capecitabine 2,500 mg/m2/day on days 1 to 14, every three weeks, for eight treatment periods. One month after surgery, the patient commenced adjuvant chemotherapy with capecitabine. At follow-up, CA 19-9 levels had returned to normal (8.6 U/mL).

The cystic mass had a circumferential muscular layer with inner circular and outer longitudinal fibers. Inset: there was focal area of in situ adenocarcinoma lining the cystic wall.
DISCUSSION
Duplications are congenital abnormalities of the gastrointestinal tract that occur rarely, but can be found anywhere from the mouth to the anus, most commonly in the ileum [1,2]. Colonic duplications comprise approximately 4-18% of all gastrointestinal duplications and are often located in the cecum [2]. In 1940, Ladd and Gross presented the following criteria for the diagnosis of duplications: intestinal duplications are vacant structures that consist of a muscular coat, usually two layers, and are lined with epithelium that resembles that of the gastrointestinal tract. These abnormalities usually extend to some portion of the alimentary tube and are tightly attached to it. The type of epithelial lining at the duplication is not necessarily consistent with that part of the gastrointestinal tract to which it is attached [4]. Our present case meets the criteria for duplication.
The pathogenesis of intestinal duplications has not yet been elucidated [1,5]. Abnormal recanalization after the solid epithelial stage of embryonic bowel development is thought by most to be the main cause of these lesions [5]. Although before surgery it is not easy to diagnose duplications of the gastrointestinal tract, differential diagnosis of a cystic mass that is located in or adjacent to the alimentary tract should include intestinal duplication [6,7]. Because intestinal duplication can induce several complications, such as bowel perforation, bleeding, obstruction, and malignant changes, proper surgical treatment should be considered [3].
Malignant change in an intestinal duplication is rarely observed [4-8]. It occurs most often in the colon, followed by the small bowel, stomach, and intrathoracic cavity [6]. Various malignant changes, such as adenocarcinoma, squamous cell carcinoma, and carcinoid tumor, have been reported [4-8]. Adenocarcinoma is the most common of these [6]. Because of the small number of patients, the prognosis of adenocarcinoma arising in an intestinal duplication is not well understood. However, some reports have suggested that cancer in small bowel duplications is more aggressive than in those cases without duplication [4].
Colonic duplication with an elevated CEA level must be considered a malignant change [7]. However, a case of elevated CA 19-9 levels in adenocarcinoma in a colonic duplication has not yet been reported. Although CA 19-9 is not a marker used in the screening of colon cancer, the prognostic value of CA 19-9 levels in colorectal cancer has been reported [9]. Accordingly, in this case, we followed up with a post-treatment analysis of CA 19-9 levels.
Adjuvant therapy for stage II colon cancer is not routinely recommended. However, high-risk patients, including those with inadequately sampled nodes, T4 lesions, perforation, poorly differentiated histology, or with lymphovascular or perineural invasion, should be considered for adjuvant chemotherapy [10]. The patient described here was deemed to have a high-risk colon cancer because lymphatic tumor emboli were present upon histologic examination. We believe that further study of the prognosis and treatment of adenocarcinoma that arises in an intestinal duplication is necessary.
Intestinal duplication is an unusual congenital anomaly, and malignant changes in these duplications are extremely rare. However, the differential diagnosis of intraperitoneal cystic lesions should include intestinal duplication, and potential malignant change of intestinal duplications should be considered.
Notes
No potential conflict of interest relevant to this article was reported.