Assessment of the Efficacy of Reducing Peginterferon Alfa-2a and Ribavirin Dose on Virologic Response in Koreans with Chronic Hepatitis C
Article information
Abstract
Background/Aims
The virologic response of Koreans to combination therapy for chronic hepatitis C is similar to westerns; however, dose modification occurs more frequently in Koreans. We evaluated the rates of peginterferon α-2a and ribavirin dose modifications and their effect on the virologic response in Koreans.
Methods
Patients with detectable HCV RNA and enrolled from multicenters were treated with peginterferon α-2a (180 µg/week) and ribavirin (800 mg/day) for 24 weeks (genotype non-1, n=37) or peginterferon α-2a (180 µg/week) and ribavirin (1,000-1,200 mg/day) for 48 weeks (genotype 1, n=55).
Results
Early virologic response (EVR) and sustained virologic response (SVR) were 77.2% (genotype 1, 75%; non-1, 81%) and 66.3% (genotype 1, 56%; non-1, 81%), respectively. The frequency of dose modification was 32.6% within the first 12 weeks and 52.2% during the entire treatment period. No difference was found in SVR regardless of dose modification. However, the SVR for patients using ≥80% of the peginterferon dose was significantly higher than for those using <80% (81.3 vs. 50.0%, p=0.007), despite varying ribavirin doses. No difference was found in SVR regardless of whether the ribavirin dose was <80% or not. These results did not change based on genotype.
Conclusions
We suggest that using at least 80% of the peginterferon α-2a dose in Koreans not only maintains SVR but also reduces drug side effects during the entire treatment period. A lower dose of ribavirin may be as efficacious as a standard dose.
INTRODUCTION
Combination therapy with peginterferon and ribavirin is the treatment of choice for patients with chronic hepatitis C virus (HCV) infection [1-3]. In westerns, this therapy produces a sustained virologic response (SVR) in approximately 40-45% of patients with HCV genotype 1 and 80% of patients with genotype non-1.
A previous report of Korean patients [4] showed efficacy for combination therapy comparable to that in studies in Western countries. In that report [4], laboratory abnormalities including neutropenia, anemia, and other adverse events such as flu-like symptoms were common events, as with non-Asian patients. Dose modification was performed in 39% (29/75) of patients without producing a significant reduction in SVR. Moreover, no difference was observed in SVR between those who received 50, 75, and 100% of the suggested peginterferon dose.
The frequency of adverse events in combination therapy is relatively high (20-64%) [1-3,5-7], and dose reductions of either peginterferon or ribavirin were required in 32-42% of patients who experienced adverse events [1-3, 5-7]. Patients experienced substantial reductions in SVR [8-9] as a result of the dose reduction. Because body weight is generally lower in Asian patients than in non-Asians, using the same dose of peginterferon in Korean patients could lead to a more frequent dose reduction and subsequently lead to a greater impairment in SVR than in non-Asian patients, although peginterferon α-2a need not be dosed by body weight [10]. The aim of this study was to evaluate the frequency of dose modification and its effect on virologic response in Korean hepatitis C patients in a routine clinical setting.
METHODS
This study stemmed from the Pegasys project, which is a multi-center, open-label expanded-access program for peginterferon α-2a (Pegasys®, Roche, Swiss) in combination with ribavirin (Viramid®, Ilsung, Korea) in Korean patients with chronic hepatitis C. The study included six clinical centers in Korea, all of which were tertiary university hospitals. The study protocol was approved by the institutional review board of each participating institution, and written consent was obtained from all patients.
Patient population
The inclusion criteria for this study were as follows: men and women aged >18 years with serologically proven chronic hepatitis C. In addition, the patients must have had detectable HCV RNA, elevated alanine aminotransferase (ALT), a liver biopsy consistent with chronic hepatitis C with or without cirrhosis, and compensated liver disease (Child-Pugh grade A). Patients with other forms of liver disease, such as active hepatitis A virus or hepatitis B virus infection, hepatocellular carcinoma, hemochromatosis, autoimmune hepatitis (antinuclear antibody positive), metabolic liver disease, alcoholic liver disease, and toxin exposures were excluded. Patients with human immunodeficiency virus, anemia, pre-existing severe depression or other psychiatric disease, seizure disorders, immunologically mediated disease, such as lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis, significant cardiac disease, chronic pulmonary disease with functional limitation, renal disease, severe retinopathy, major organ transplantation with an existing functional graft, thyroid disease poorly controlled on prescribed medications, and drug abuse (including excessive alcohol consumption) were also excluded.
Treatment protocol
Patients with genotype 1 were treated with peginterferon α-2a 180 µg/week and a daily ribavirin dose of 1,000 mg (weight <75 kg) or 1,200 mg (weight ≥75 kg) for 48 weeks. Alternatively, patients with genotype non-1 were treated with peginterferon α-2a 180 µg/week and a daily ribavirin dose of 800 mg for 24 weeks. Patients with no early virologic response (EVR) at week 12 were recommended to stop treatment at that time. However, continued treatment with peginterferon α-2a may be justified in individual patients, particularly those who have accompanying cirrhosis and could benefit from an improvement in histology. Complete blood cell counts and blood chemistry were monitored at screening and at weeks 2, 4, 8, 16, and 24 for both genotypes and at weeks 32 and 48 for genotype 1 only. In addition, HCV RNA was checked at weeks 12, 24, and 48 for both genotypes and at week 72 for genotype 1 only.
Definitions of response
EVR was defined as the absence of detectable serum HCV-RNA by a Roche AMPLICOR HCV Test v2.0 (Roche Molecular Systems; Pleasanton, CA, USA) (<50 IU/mL) or a decrease in HCV-RNA of at least log 2 after 12 weeks of treatment. SVR was defined as the absence of detectable serum HCV RNA after 24 weeks of treatment.
Dose modification
Dose modification included a dose reduction or premature discontinuation of treatment. The peginterferon α-2a dose was reduced stepwise from 180 to 135, to 90, and then to 45 µg/week if the absolute neutrophil count (ANC) fell below 750 cells/mm3. For patients with an ANC below 500 cells/mm3, treatment was suspended until ANC values returned to more than 1,000 cells/mm3. A dose reduction of peginterferon α-2a to 90 µg was recommended if the platelet count fell below 50,000 cells/mm3. Cessation of therapy was recommended when the platelet count decreased to levels below 25,000 cells/mm3. The dose was initially reduced to 90 µg if ALT progressively increased above baseline values. The normal upper limit for ALT was defined as 45 U/mL. When an increase in ALT levels was progressive, despite dose reduction, or was accompanied by increased bilirubin or evidence of hepatic decompensation, therapy was discontinued.
The daily ribavirin dose was reduced by 200 mg when a patient without significant cardiovascular disease experienced a drop in hemoglobin to 8.5-10 g/dL or a patient with stable cardiovascular disease experienced a drop in hemoglobin by >2 g/dL during any 4 weeks of treatment. Ribavirin was discontinued if a patient without significant cardiovascular disease experienced a decrease in hemoglobin to a level confirmed to be below 8.5 g/dL or if a patient with stable cardiovascular disease maintained a hemoglobin value <12 g/dL despite 4 weeks on a reduced dose of 600 mg. Further dose modification of one or both drugs for other adverse events was based on side-effect severity. The dose of ribavirin was increased if the hemoglobin increased above 10 g/dL or if the adverse event resolved. In general, we adjusted and maximized ribavirin doses according to tolerability [11].
The treatment period for any patient was not extended beyond that specified for his/her assigned treatment group (i.e., 24 weeks for genotype non-1 and 48 weeks for genotype 1).
Dose counting
The doses of each drug taken by each patient were measured during the course of the study and evaluated by reviewing the case report form. The amounts of both medications taken by each patient during the first 12 weeks of treatment were expressed as a percentage of the total prescribed dose. The percentage of the total dose of each drug received during the entire treatment period was calculated in a similar fashion.
Statistical analysis
The statistical analysis for the virologic response was performed using an intention-to-treat analysis. Basal characteristics were expressed as means±2SD or the absolute number and percentage of patients. ANOVA, Pearson chi-square test, or Fisher's exact test were used to compare means or the proportion of basal characteristics between two or more groups. The trends within each dose category were determined with a linear-by-linear association. The factors affecting SVR were analyzed by logistic regression. All analyses were two-tailed with an α of 0.05. All calculations were performed with SPSS version 14 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient population
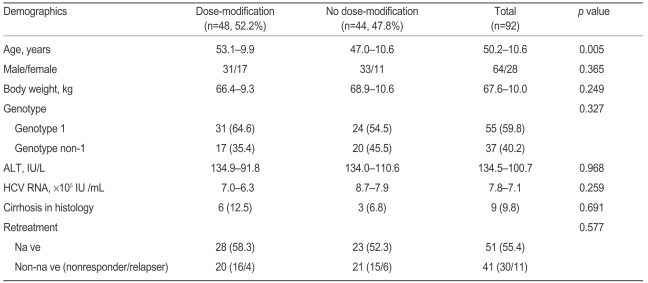
A total of 92 patients were enrolled in this study (Table 1). Thirty-seven patients were infected with genotype non-1 and 55 patients with genotype 1 hepatitis C. The mean age of the patients was 50.2 years, and the sex ratio (male/female) was 64/28. The mean body weight was 67.6 kg, and the mean serum ALT and HCV RNA were 134.5 IU/L and 7.8 × 105 IU/mL, respectively. All patients underwent a liver biopsy, and the results of the histologic examinations revealed a 9.8% occurrence of liver cirrhosis. The percentage of naïve patients was 55.4% (51/92), and the proportions of naïve patients in genotype 1 and non-1 were 47.3% and 67.6%, respectively (not shown in Table 1). Among non-naïve patients, 30 were non-responders and the remaining 11 patients had relapsed. Non-naïve patients had been injected with conventional interferon.
After checking the EVR of all patients at week 12, we excluded three patients in genotype non-1 and four patients in genotype 1 due to EVR failure. One patient with genotype 1 was excluded due to severe anemia and neutropenia at week 27. Two patients with genotype 1 were lost from follow-up after treatment week 48 (Fig. 1). Two patients with genotype non-1 discontinued the ribavirin (one each at weeks 6 and 8), but maintained the peginterferon alone over the entire treatment period (not shown in the Fig. 1).

Patient flow diagram. Three patients in genotype non-1 and four patients in genotype 1 were excluded from the study due to EVR failure. One patient with genotype 1 was excluded due to adverse events at week 27, and two patients were lost to follow-up after the end of treatment before SVR was checked. EVR, early virologic response; SVR, sustained virologic response.
A dose modification was performed in 32.6% of patients within the first 12 weeks of treatment and in 52.2% of patients during the entire treatment period (Table 1). The mean age of patients in the dose-modification group was significantly higher than that in the no dose-modification group. However, in cases of genotype 1 patients who should have received more ribavirin than did genotype non-1 patients, no significant difference was found in the dose-modification rate compared to genotype non-1 patients. Forty-one of the 55 patients (74.5%) in the genotype 1 group received a 1,000 mg dose of ribavirin due to low weight (<75 kg). Among the genotype 1 patients, the rate of dose modification was not different between 1,000 mg and 1,200 mg users (59.5% vs. 50.0%, p=0.579). The fraction of naïve and non-naïve patients, proportion with cirrhosis, sex ratio, body weight, mean serum ALT level, and HCV RNA were not different between the dose-modification and no dose-modification groups.
Virologic response rates according to dose modification
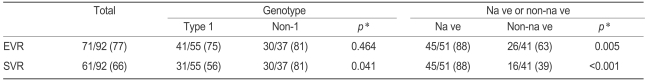
The EVR and SVR of all patients evaluated were 77% and 66%, respectively (Table 2). EVR was not significantly different between the HCV genotypes. The SVR of genotype non-1 patients was significantly higher than that of the genotype 1 group (81% vs. 56%, p=0.041). The EVR and SVR of the naïve patients were significantly higher than were those of the non-naïve patients (88% vs. 63%, p=0.005 and 88% vs. 39%, p<0.001, respectively).
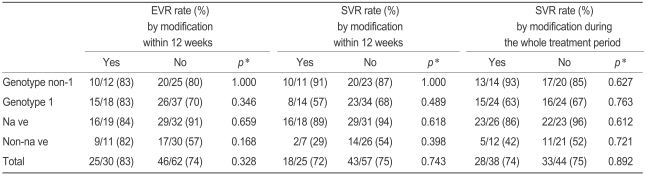
However, no difference was found in EVR and SVR regardless of dose modification and regardless of whether the modification occurred within the first 12 weeks or at any time during the treatment period. Also, this result held regardless of genotype and of whether the patient was drug-naïve. Similarly, EVR did not significantly change after dose modifications in the first 12 weeks in either genotype group or in the naïve and non-naïve groups (Table 3).
The effect of reducing peginterferon or ribavirin dose on SVR during the entire treatment period
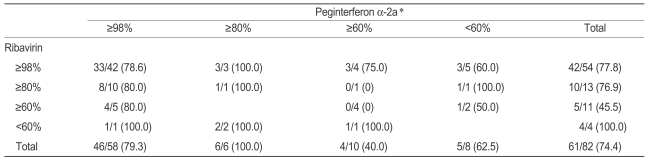
We analyzed the impact of reducing the peginterferon dose independent of ribavirin. The effect of reducing the dose of one or both drugs on SVR is described in Table 4. SVR was not adversely affected when the dose of ribavirin was reduced from 98-100% to ≤60% (p=0.832) in patients who received a full dose of peginterferon. A comparison of the SVR from the four peginterferon groups revealed a strong relationship between reducing the peginterferon dose and impaired SVR (p=0.050). A comparison of the SVR from the four ribavirin groups revealed no relationship between reductions in the ribavirin dose and impaired SVR (p=0.488).
We separated and regrouped the four peginterferon groups into two groups on either side of the 80% peginterferon dose level and found that SVR was significantly higher for those using ≥80% of the peginterferon dose compared to those using <80% of the peginterferon dose (p=0.007). Using the same method and regrouping the four ribavirin groups on either side of the 80% ribavirin dose revealed no significant difference in SVR between the two ribavirin-dose groups (p=0.158). We also compared the effect of using ≥80% of the peginterferon dose between the genotype 1 and non-1 groups and between the naïve and non-naïve patients. Results indicated that SVR for those using ≥80% of the peginterferon dose was significantly higher than that for those using <80% of the peginterferon dose in the genotype 1, naïve, and non-naïve groups, but it was not significantly different in the genotype non-1 group. No difference was found in SVR in either genotype group or in the naïve group based on using 80%≥ or <80% of the ribavirin dose. In the non-naïve patients, SVR was higher for those using ≥80% of the ribavirin dose than for those using <80% of the dose (p=0.044). However, the number of patients using <80% of the ribavirin dose was too small to analyze.
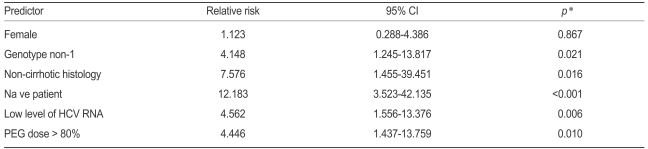
We also analyzed several SVR predictors, adjusted for age and body weight in logistic regression models (Table 5). The results indicated that using more than 80% of the peginterferon dose was a significant predictor (relative risk [RR], 4.446; confidence interval [CI], 1.437-13.759) of SVR. Other variables including genotype non-1, no cirrhosis in histology, drug naïve, and low HCV RNA level were also significant predictors of SVR.
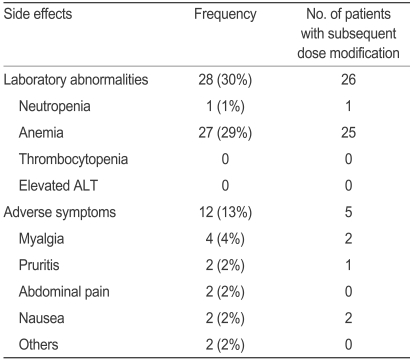
Side effects
The side effects of peginterferon and ribavirin are presented in Tables 6 and 7. The main causes for dose modification in the peginterferon and ribavirin groups were neutropenia and anemia, respectively. Among the patients who showed laboratory abnormalities, over 90% reduced the dose or stopped taking the drug. We found that 12 patients in both the peginterferon and ribavirin groups complained of adverse events such as myalgia and pruritis, among others. Among the patients who experienced adverse events, 50% reduced the dose or stopped taking the drugs.
DISCUSSION
This study analyzed the effect of peginterferon and ribavirin dose modification on SVR in Korean patients with chronic hepatitis C. In another Korean report [4], Korean patients were similar to non-Asian patients in their virologic response to combination therapy [1-3]; however, the doses were modified in over half of the patients during the treatment period.
The rate of dose modification appears to be higher in Asian patients than in non-Asian patients. In Western studies, dose modification and premature discontinuation for adverse events were found in 32 and 10% of patients, respectively [1,2]. In Japanese and Taiwanese studies, 56 and 46% of patients, respectively, required a dose modification [12,13]. Considering the basal characteristics of enrolled patients, we found a difference in age and body weight between Asians and non-Asians. In Japan, patients infected with chronic hepatitis C who are currently being treated with interferon are 10-15 years older on average than are corresponding patients in the United States, where patients treated with antiviral therapy tend to average 45 years of age [12]. In that study, dose modification was more frequent in older groups, occurring in 38, 42, and 77% of the <50, 50-59, and ≥60 year-old groups, respectively (p<0.001). On average, patients in present study were 5 years older than were the Western patients, and the mean age in the dose modification group was higher than that in the no dose modification group (p=0.005). This suggests that age is a significant factor for impaired adherence and affects the safety of combination therapy. Furthermore, the body weight of patients in the Asian studies is lower than that of patients in the Western studies (about 65 vs. 80 kg) [1-4, 12,13]. Although peginterferon α-2a dose need not be altered for body weight [10], the lower body weight in Asian patients is believed to contribute to a higher dose modification rate for peginterferon α-2a.
We found that simple dose modification and the timing of dose modification had no significant effect on EVR and SVR. Although one limitation of this study is the heterogeneity of the patient population with regard to genotypes and prior drug experience, this result was observed for all subgroups. We also found that using ≥80% dose of peginterferon was a significantly affected SVR, despite variations in the ribavirin dose. Moreover, this factor was significant in all subgroups except for the genotype non-1 group. This result is consistent with a previous report indicating that genotype non-1 is a good prognostic factor for achieving SVR. Nevertheless, our results suggest that more than 144 µg/week (80% of 180 µg/week) of peginterferon α-2a during the entire treatment period may be the optimal dose for Korean patients.
In contrast to our results, several studies have suggested that reducing the dose, especially in the first 12 weeks of treatment, is associated with a decline in SVR [8-9,12,14-16]. In the HALT-C trial cohort, the SVR of patients dropped from 17 to 7% when the peginterferon dose was reduced during the initial 20 weeks of therapy [14]. A reduction from ≥80% to ≤60% of the planned total dose during the first 20 weeks of treatment was associated with a decline in SVR from 20-21% to 11-13% [9]. However, these studies were based on non-responder retreatments or peginterferon α-2b (1.5 µg/kg/week). Another study found no significant relationship between the timing of dose reduction and impaired SVR, similar to our study [8]. In the present study, most patients who reduced the dose within the first 12 weeks did not discontinue the drugs; rather, the drug doses were adjusted and maximized according to tolerability [11]. This may be the reason that reducing the drug dose had no effect on SVR within the first 12 weeks.
Reducing or discontinuing ribavirin prematurely was associated with a marked drop in SVR [14-15,17-19]. However, in a large long-term trial from the HALT-C trial cohort [14], it was suggested that reducing the ribavirin dose appeared to have little impact on SVR if patients did not discontinue ribavirin and remained on full-dose peginterferon. This is consistent with our result, although we could not analyze the effect on SVR of discontinuing ribavirin. Considering the older age and lower body weight among patients in the present study than among Western patients [1-3], a large number of patients could be permitted the lower dose of ribavirin; thus, 75% of patients could take more than 80% and most could take more than 60% of the ribavirin. This may be the reason that the ribavirin dose had no significant effect on efficacy in our study, as in other studies. Moreover, two patients with genotype non-1 prematurely discontinued ribavirin at weeks 6 and 8 but achieved EVR and SVR by maintaining the full dose of peginterferon alone. Although further study with a large, homogenous group is necessary, we suggest that the effect of ribavirin on SVR was less apparent than was that of peginterferon.
Side effects, especially hematologic toxicity, accounted for the vast majority of dose reductions [1,2]. Analysis of basal characteristics that could lead to more side effects showed that older age and lower body weight were more significant factors than was the drug itself.
Several recent studies have examined whether the treatment period can be shortened [20,21]. These studies indicate that treatment duration for HCV genotype non-1 should be shortened to less than 24 weeks if the patient achieves a rapid virologic response (RVR). In the present study, we did not check RVR at week 4, but analyzed the percentage of total prescribed dose and examined the impact of reducing the peginterferon dose according to the timing of dose modification instead of shortening the treatment period based on RVR.
In conclusion, the present analyses have enhanced our understanding of how dose reduction can affect SVR in Korean patients with chronic hepatitis C. We suggest that using at least 80% (144 of 180 µg/week) of the peginterferon α-2a during the entire treatment period not only maintains SVR but also reduces side effects. In addition, our results show that using a dose of ribavirin lower than the standard dose may still be efficacious in Korean patients. Further studies should be conducted in larger populations with a homogeneous genotype to confirm these results.
Notes
This study was supported by a grant of the Korea Centers for Disease Control and Prevention (2007-S3-B-001) and the Songeui Foundation of the Catholic University of Korea for Medical Research.