Endoscopic and Histopathologic Predictors of Recurrence of Colorectal Adenoma on Lowering the Miss Rate
Article information
Abstract
Background/Aims
Although colorectal adenoma is reported to recur frequently, this may result from missing it at baseline. However, few studies of recurrence have considered the miss rate. This study evaluated the recurrence rate prospectively and clinical predictors of recurrence in colorectal adenoma after lowering the miss rate.
Methods
The study population comprised 128 patients who underwent baseline colonoscopy with resection of colorectal adenomas. Re-examination to lower the miss rate was performed within 2 months. Follow-up colonoscopy to detect recurrence was done more than 1 year after removal.
Results
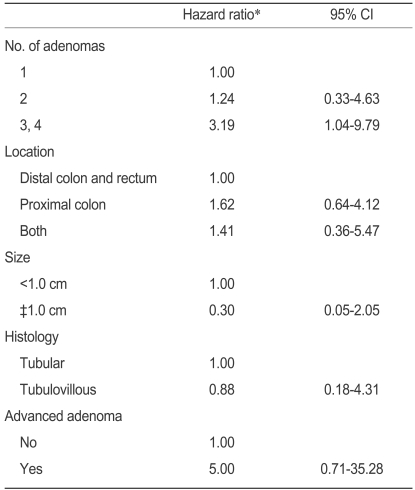
The mean follow-up period was 35.1 months (range, 12 to 84 months). Thirty patients had a recurrent adenoma, for a recurrence rate of 23.4%. Older patients (over 60 years) had a two-fold greater risk of recurrence than younger patients (hazard ratio, 2.39; 95% confidence interval [CI], 1.16-4.90). Patients with three or four adenomas at baseline colonoscopy had a two-fold greater risk than those with one adenoma (hazard ratio, 2.44; 95% CI, 1.11-5.35). Patients with advanced adenoma had a two-fold greater risk than those with no advanced adenoma (hazard ratio, 2.88; 95% CI, 1.40-5.95). In multivariate analysis, only the presence of three or four adenomas independently predicted the recurrence of adenoma (hazard ratio, 3.19; 95% CI, 1.04-9.79).
Conclusions
The recurrence rate of colorectal adenoma corrected by lowering the miss rate was lower than reported rates. The presence of multiple adenomas on initial colonoscopy was an important predictor of recurrence.
INTRODUCTION
Most colorectal cancers arise from adenomatous polyps. Colorectal adenomas are common in the general population. The estimated prevalence rate is 30-50% after the age of 65 years [1-3]. The removal of adenomas is associated with a lower risk of colorectal cancer [4,5]. However, patients with removed adenomas remain at high risk of developing new adenomas or cancers, justifying follow-up with repeated colonoscopies [6]. The clinical characteristics of patients at high risk of adenoma recurrence are controversial.
The main problem in studying adenoma recurrence is a falsely high estimated recurrence rate due to missing adenomas at the time of the baseline colonoscopy. The estimated colonoscopic miss rate for polyps is 15-24% [7-9]. Most research on adenoma recurrence and risk factors has not considered the miss rate. Therefore, this study evaluated the recurrence rate of colorectal adenoma prospectively, and the clinical characteristics associated with recurrence when the miss rate was reduced by follow-up colonoscopy within 2 months.
METHODS
Study population
The study enrolled 667 patients who were older than 40 years, underwent colonoscopy for the first time between March 1997 and June 2001, and had a colorectal adenoma or mucosal cancer that was diagnosed on pathology after polypectomy. We excluded 119 patients with advanced cancer, polyposis syndrome, inflammatory bowel disease, intestinal tuberculosis, and five or more polyps. Of the 548 patients, 232 patients underwent a re-examination to detect and remove missed adenomas within 2 months. Ultimately, the study population comprised 128 of the 232 patients, who underwent a follow-up colonoscopy after 1 year or more.
Endoscopic procedures
Colonoscopy was performed with a standard colonoscope (CF-Q240AL, Olympus Optical, Tokyo, Japan) by three experienced endoscopists who had each performed more than 500 cases annually for at least 3 years. All patients were given a routine bowel preparation that included the ingestion of 4 L of a balanced electrolyte solution with polyethylene glycol 6 hours before the procedure. The degree of bowel preparation was classified as good, acceptable, and poor. Only good bowel preparation was included in this study. Meperidine (50 mg) and midazolam (3 mg) were administered to all patients just before colonoscopy. To increase the polyp detection rate, 0.2% indigo carmine was sprayed on suspected lesions. Colonoscopic withdrawal was done as slowly as possible over 6 minutes.
We analyzed the number, size, location, and morphology of adenomatous polyps at baseline colonoscopy and the existence of villous architecture and degree of dysplasia on pathology. Advanced adenoma was defined as an adenoma of diameter ≥10 mm or a villous component or severe dysplasia. The location of the adenoma was divided into the proximal colon (the cecum, ascending, and transverse colon) and the distal colon (the splenic flexure, descending colon, sigmoid colon, and rectum). For patients with multiple adenomas, the most affected adenoma was used to classify the size, location, morphology, and histologic architecture. The most affected adenoma was defined as mucosal cancer first, advanced adenoma second, and then the largest adenoma.
The polyp miss rate was defined as the proportion of polyps detected only during the second colonoscopy relative to the total number of polyps found during the first and second examinations.
Follow-up colonoscopy was performed at least 1 year after the baseline colonoscopy to evaluate recurrent adenomas, which were assessed according to number, size, location, morphology, villous component, degree of dysplasia, and existence of advanced adenoma. The primary end point was adenoma recurrence. The annual recurrence rate was calculated as the number of patients with recurrent adenoma in a given year divided by the number of patients who underwent colonoscopy in the same period.
The study was approved by our institutional ethics committee and written informed consent was obtained from each patient before the procedure.
Statistical analysis
Standard procedures in the Statistical Package for the SPSS version 11.5 (SPSS Inc., Chicago, IL, USA) were used for the statistical analysis. Kaplan-Meier analyses with log-rank test were used to identify univariate predictors of adenoma recurrence. Multivariate analysis was conducted using Cox proportional hazard model. The importance of each independent variable was summarized by its hazard ratio and 95% confidence interval (CI). Kaplan-Meier curves were used to estimate the cumulative recurrence rates, and the log-rank test was applied to compare differences between cumulative recurrence curves. The chi-square test was used to compare the characteristics of baseline and recurrent adenomas and the miss rate of polyps according to the initial number and size of polyps. A p value less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Of the 128 patients, 66 were male and 62 were female. The mean age was 55.1±0.8 years (mean±standard error of the mean; range 40-81). The mean age of the recurrence group was 57.5±1.4 years (range 41-71), and that of the non-recurrence group was 54.3±0.9 years (range 40-81). The mean follow-up period was 35.1±1.6 months (range 12-84).
Miss rate of polyps and adenomas
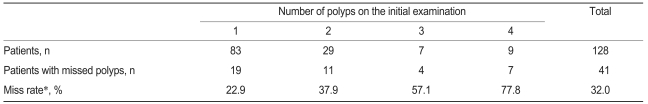
During the first and second examinations, a total 250 polyps were found, 53 of which were missed. The overall miss rate of polyps was 21.2%. The miss rate of colorectal polyps based on the initial number of polyps is shown in Table 1. As the number of polyps found during the initial examination increased, the miss rate increased significantly (p=0.001) and was 22.9, 37.9, 57.1, and 77.8% in patients with one to four polyps, respectively. The miss rate based on polyp size was 23.9% for 1-4 mm polyps, 19.8% for 5-9 mm polyps, and 10% for polyps ≥10 mm (Table 2). The polyp miss rate tended to be greater with smaller size, but the effect was not significant (p=0.159). At the first and second examinations, a total of 214 adenomas were found, of which 38 were missed for an overall miss rate of 17.7%.
Adenoma recurrence rate
A total of 30 of the 128 patients had recurrent adenomas and the recurrence rate was 23.4%. The number of patients examined during each year of follow-up was 69, 35, 32, 24, 8, and 5 in years 1-2, 2-3, 3-4, 4-5, 5-6, and 6-7, respectively, for respective annual recurrence rates of 27.5, 14.3, 12.5, 4.2, 0, and 20% (Fig. 1). In four patients, the recurrence was an advanced adenoma: three were found in year 1-2 and one in year 2-3.
Patient characteristics associated with recurrence
The older group (over 60 years) had a two-fold greater risk than the younger group (hazard ratio, 2.39; 95% CI, 1.16-4.90). There was no significant difference according to sex or family history of colorectal cancer (Table 3).
Adenoma characteristics associated with recurrence
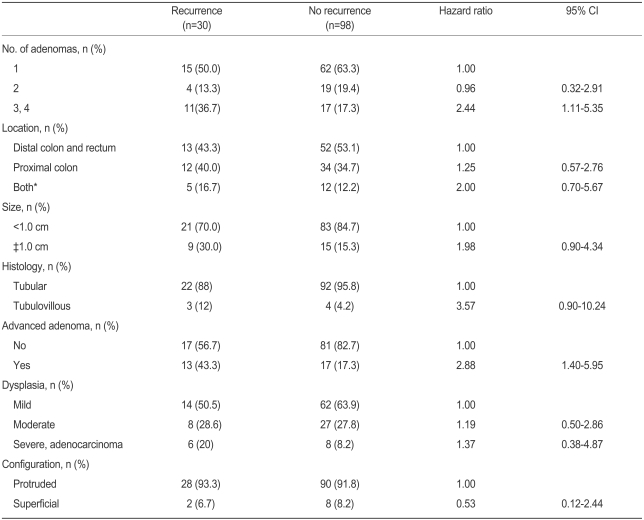
Patients with three or four adenomas at baseline colonoscopy had a two-fold greater risk of recurrence than those with one adenoma (Table 4, hazard ratio, 2.44; 95% CI, 1.11-5.35). Patients with advanced adenoma had a two-fold greater risk than those without (hazard ratio, 2.88; 95%CI, 1.40-5.95). Although not statistically significant, patients with a large adenoma (≥1 cm in diameter) or a tubulovillous adenoma tended to have a greater risk than those with a small adenoma (hazard ratio, 1.98; 95% CI, 0.90-4.34) or a tubular adenoma (hazard ratio, 3.57; 95% CI, 0.90-10.24). There was no difference in recurrence rate associated with the location, morphology, or degree of dysplasia. On multivariate analysis, patients with three or four adenomas had a three-fold greater risk of recurrence than those with one adenoma (hazard ratio, 3.19; 95% CI, 1.04-9.79). No other variable had a significant effect (Table 5). Figure 2 summarizes the cumulative adenoma recurrence rate according to the number of adenomas and presence of advanced adenoma.
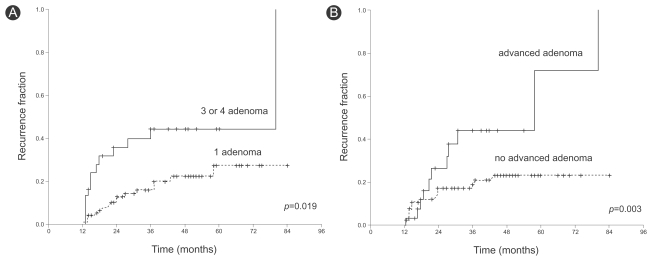
The cumulative adenoma recurrence rate according to the number of adenomas and presence of advanced adenoma. (A) Patients with three or four adenomas at baseline colonoscopy had a greater recurrence rate than those with one adenoma. (B) Patients with an advanced adenoma had greater recurrence than those without.
DISCUSSION
The adenoma carcinoma sequence is a slow, multistep process, and only a small proportion of adenomas, approximately 3 to 5%, develops into carcinoma after 10 years [10]. Colonoscopy studies have observed colorectal adenomas in 40% of the population over 50 years of age [11,12]. Primary prevention of colorectal cancer is limited because the causes of this ailment are unclear. Secondary or tertiary prevention, i.e., the removal of precancerous lesions or early cancers, may be more useful for reducing mortality due to colorectal cancer.
Most observational studies have revealed a substantial reduction in the risk of colorectal cancer after adenoma removal. However, patients with removed adenomas are recommended to undergo repeat colonoscopy due to the high risk of recurrence. With the cost and complications of colonoscopy, the main challenges are to formulate guidelines for follow-up duration and define the groups of patients who need intense follow-up [6].
The main problem with recurrence studies lies in the high miss rate of polyps during colonoscopy, which leads to falsely high recurrence rates. Most studies of adenoma recurrence have reported recurrence rates and risk factors without considering the colonoscopic miss rate. There is evidence that the miss rate is higher in cases with multiple polyps, small polyps, or polyps in the right colon [7,8]. In a meta-analysis of 465 patients in 6 studies by van Rijn et al. [9], the estimated overall miss rate was 22%: 26% in patients with 1-5 mm polyps, 13% for 5-9 mm polyps, and 2.1% for polyps larger than 10 mm.
In the present study, to reduce the miss rate, we excluded patients with five or more polyps and performed a follow-up colonoscopy within 2 months to identify and remove missed polyps. The reported miss rate is higher in cases with multiple polyps [8]. This tendency was also seen in our study when multiplicity was limited to less than five polyps. We excluded patients with five or more polyps to reduce the miss rate because missed adenomas may be considered recurrent ones, even though we recognize that this exclusion creates a slight selection bias that somewhat limits the study results. The mean follow-up period was 35.1 months (range 12-84) and the recurrence rate was 23.4%, which was generally lower than the 22-50% reported in previous studies [6,13-23]. We believe that this resulted from correcting the recurrence rate by lowering the miss rate by follow-up colonoscopy performed within 2 months.
Although re-examination at a short interval is not performed in routine clinical practice, the institutional ethics committee approved the method, as it was recognized as essential to the purpose of this study.
When to undergo a follow-up colonoscopy after removing an adenoma is controversial. Follow-up colonoscopy in patients with 1 or 2 small (<1 cm) resected adenomas is recommended in 5-10 years, depending on the institute. Follow-up colonoscopy in patients with multiple adenomas or an advanced adenoma is recommended within 3 years in most institutes [24-26]. According to the annual recurrence rates in our study, most recurrent adenomas developed within 3 years and all four advanced adenomas did so within 3 years. Hence, in our opinion, follow-up colonoscopy should be performed within 3 years.
Predictors of colorectal adenoma recurrence have not been well established. The reported risk factors for recurrence include multiple adenomas [6,13-23], a large adenoma [17,19,20,22], severe dysplasia [13,20], a tubulovillous/villous adenoma [13,20,23], and an adenoma in the proximal colon [6,14]. In our study, the recurrence of colorectal adenoma was significantly greater in patients with three or four adenomas and advanced adenoma. Although not statistically significant, patients with a large adenoma (≥10 mm in diameter) or a tubulovillous adenoma tended to have a greater risk of recurrence. As this study examined relatively few cases, other characteristics of polyps, such as size or histology, may not have reached statistical significance. On multivariate analysis, patients with three or four adenomas had a three-fold greater risk of recurrence. Therefore, the presence of multiple adenomas independently predicts the recurrence of adenoma.
Some studies have reported that recurrent adenomas tended to be multiple, small, tubular, and in the proximal colon compared with the baseline adenomas [14]. In contrast, we found that all recurrent adenomas had a tubular architecture and most were single lesions. There was no difference in the size or location of the recurrent adenomas. Perhaps the reason why most recurrent adenomas were single lesions is that the missed polyps were identified at follow-up colonoscopy performed within 2 months.
In conclusion, the recurrence rate of colorectal adenoma corrected by lowering the miss rate (i.e., 23.4%) was lower than that in most previous reports. We found that the presence of multiple adenomas at baseline colonoscopy was an important predictor of the recurrence of colorectal adenoma. Therefore, a more careful follow-up examination should be performed in patients with multiple adenomas at baseline colonoscopy.