Comparison of the Applicability of Two Prognostic Scoring Systems In Patients with Fulminant Hepatic Failure
Article information
Abstract
Background
Distinguishing those patients with fulminant hepatic failure (FHF) and who require transplantation from those FHF patients who will survive with receiving only intensive medical care remains problematic, and this distinction is important because of the chronic shortage of donor livers.
Methods
To assess the applicability of two prognostic scoring systems, referred to as the London and Clichy criteria, we compared using both systems, at the time of admission, for 43 FHF patients (15 M/28 F; age: 3716 yrs). Acetaminophen (ACM) was the etiology for 16 patients, while the remaining 27 had other etiologies. All the patients received intensive care, and 18 (8 ACM/10 non-ACM) had investigational BAL support.
Results
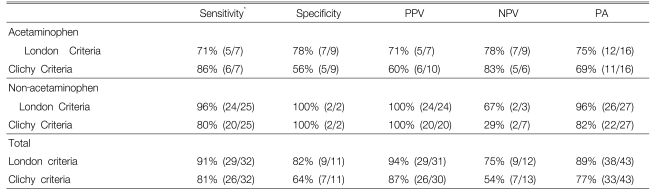
For the ACM toxicity, neither the London nor the Clichy criteria exhibited acceptable sensitivity (71 vs 86%, respectively), specificity (78 vs 56%, respectively), a positive predictive value (71 vs 60%, respectively), a negative predictive value (78 vs 83%, respectively) or predictive accuracy (75 vs 69%, respectively) to predict patient survival without transplantation. In contrast, applying the London and Clichy criteria to the FHF patients with non-ACM etiologies showed a sensitivity of 96 vs 80%, respectively, a specificity of 100 vs 100%, respectively, a positive predictive value of 100 vs 100%,, respectively a negative predictive value of 67 vs 29%, respectively and a predictive accuracy of 96% vs 82%, respectively.
Conclusions
Overall, the London criteria more accurately predicted the need for transplantation, and neither the London criteria nor the Clichy prognostic criteria accurately predicted the outcome of those patients who suffered with FHF due to ACM. BAL support may have contributed to the survival of the patients with ACM toxicity and who didn't undergo transplantation, and this survival exceeded the predictions of both prognostic systems. Additional multicenter studies should be conducted to refine these prognostic scoring systems, and this will help physicians rapidly identify those FHF patients who can survive without undergoing liver transplantation.
INTRODUCTION
Fulminant hepatic failure (FHF) was originally defined by Trey and Davidson in 1970 as the occurrence of hepatic encephalopathy within 8 weeks of the onset of acute liver disease in the absence of chronic liver disease1). Several clinical variants of FHF, based on the interval between the onset of jaundice and encephalopathy, were subsequently proposed, and these differed with respect to the etiology, the risk of cerebral edema and the prognosis for survival with administering medical therapy alone2-5). The original classifications of both O'Grady et al.6) and Bernau et al.2) omitted the requirement for the absence of prior hepatic disease and the classifications included those patients with FHF syndrome and who had pre-existing asymptomatic liver diseases.
An estimated 2000 patients per year develop FHF in the United States7). Despite the advances in critical care management, medical therapy alone has resulted in overall survival rates of only 10~40% for patients suffering with FHF and who progress to stage III or IV hepatic encephalopathy7). Liver transplantation (LT) represents the only lifesaving therapy for patients with progressive FHF and this has increased the survival rates to 60~80%8, 9).
Distinguishing the patients with FHF who require LT from those patients who will likely survive with intensive medical care alone remains problematic, but this is increasingly important due to a critical shortage of donor livers. Although two prognostic scoring systems, referred to as the London10, 11) and Clichy12-15) criteria, have been used to assess European patients with FHF, only one transplant center in the U.S.A.16) has assessed the applicability of the London scoring system. The aim of the present study was to compare the sensitivity, specificity, the positive and negative predictive power and the predictive accuracy of both the London and Clichy prognostic scores for patients with FHF.
MATERIALS AND METHODS
Patients
Between June 1990 and April 1999, 94 patients with FHF were admitted to the Liver Support Unit of Cedars-Sinai Medical Center for diagnosis and treatment. Both the London and Clichy prognostic scoring systems were used to access 43 patients on admission. The other 51 patients were ineligible because they had received transfusions of fresh frozen plasma prior to admission or that the measurement of factor V activity with using the available frozen serum would have yielded unreliable results17).
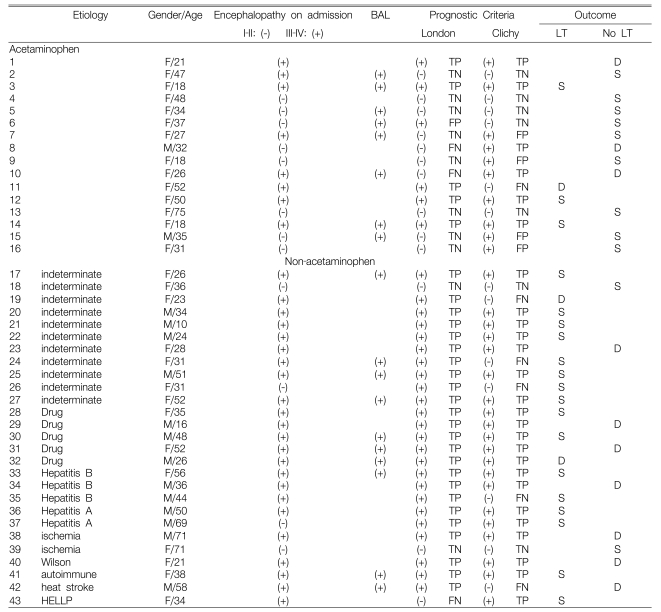
The clinical characteristics and outcomes of the 43 patients are summarized in Table 1. The mean age of the 43 patients (28 females and 15 males) was 37 years (range: 10 to 75 years). Acetaminophen (ACM) toxicity was the etiology of FHF for 16 of 43 patients (37%). Non-acetaminophen (NACM) etiologies were identified for 27 patients (63%): they were indeterminate (n=11), other hepatotoxic drugs (n=5), hepatitis B (n=3); hepatitis A (n=2), ischemia (n=2), heat stroke (n=1), autoimmune hepatitis (n=1), Wilson's disease (n=1), hemolysis, elevated liver enzymes and low platelet (HELLP) syndrome (n=1).
At the time of transfer to our hospital, 8 of 16 (50%) patients with ACM toxicity and who had grade III or IV hepatic encephalopathy were intubated or they required urgent intubation upon admission. Among the 27 patients with NACM etiologies of FHF, 23 (85%) were admitted with grade III or IV hepatic encephalopathy. All the patients were treated in a specialized intensive care unit by a multidisciplinary team of hepatologists, transplant surgeons, intensive care specialists and consultants. Eighteen patients (8 with ACM and 10 with NACM etiologies), underwent one or more treatments with experimental bioartificial liver (BAL) support (Table 1) under a protocol that as been previously described18, 19).
Prognostic Scoring
The static and dynamic information required for evaluating both the London and Clichy criteria were compared on admission. The London prognostic criteria that were specific for patients with the ACM and NACM etiologies of FHF were used10). The specific criteria for a poor prognosis for the patients with ACM-induced FHF included: 1) an arterial blood pH <7.30 regardless of the stage of hepatic encephalopathy or 2) the constellation of grade III or IV hepatic encephalopathy with a PT of >100 sec. (which corresponds in our laboratory to a PT INR of >6.5) and a serum creatinine level >3.4 mg/dL. The adverse prognostic criteria for the patients with the NACM etiologies of FHF in the London scoring system10), which are independent of the stage of hepatic encephalopathy, included: 1) a PT INR >6.5 or 2) at least 3 of the 5 following indicators: a) age <10 or >40 years; b) indeterminate, halothane or non-acetaminophen drug etiologies; c) the interval between jaundice and the onset of hepatic encephalopathy >7 days; d) a PT INR >3.5; or e) a serum bilirubin level >17.5 mg/dL.
The Clichy criteria included the stages I-IV hepatic encephalopathy and a factor V activity of <20% in the patients <30 years of age or <30% in the patients >30 years of age15). The factor V activity was measured in the serum obtained on admission by the Cedars-Sinai clinical laboratory with using a standard one-stage clotting technique. Frozen plasma was not used to avoid underestimation of the true factor V activity17). Patients were also excluded if they had received a fresh frozen plasma transfusion prior to admission.
Data Analysis
The sensitivity, specificity, positive predictive value, negative predictive value and accuracy were calculated to compare the predictive value of the London and Clichy criteria20). A true positive outcome was defined as a patient who met the criteria for LT and who either died with recieving medical therapy or they underwent LT. A true negative outcome was defined as a patient who didn't meet the criteria for LT and who survived with receiving medical therapy alone. Sensitivity was defined as the number of true positive outcomes divided by the total number of patients who died or underwent LT. Specificity was calculated as the number of true negative outcomes divided by the total number of patients who survived without LT. The positive predictive value (PPV) was defined as the ratio of the true positives to the combined true and false positives. The negative predictive value (NPV) was defined as the ratio of true negatives to the combined true and false negatives. The predictive accuracy (PA) was defined as the ratio of the combined true positives and negatives to the total number of patients.
RESULTS
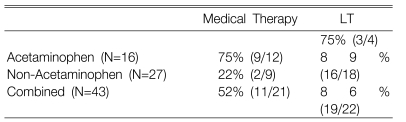
Table 1 summarizes the clinical features and outcomes of all 43 patients. Eleven of the 43 patients (26%) survived with receiving medical therapy alone. The survivors included 9 of 16 (56%) patients with ACM etiologies and 2 of 27 (7%) patients with NACM etiologies. Among the survivors who received medical therapy alone, 5 of the 9 patients (56%) in the ACM group and 0 of 2 in the NACM group had been treated with BAL support. Ten of 43 patients (23%) died during medical therapy, including 3 of 16 (19%) in the ACM group and 7 of 27 (26%) in the NACM group. Among those patients who died without LT, BAL support was used for one patient in the ACM group and for 2 patients in the NACM group. Twenty-two of the 43 patients (51%) that were transplanted included 4 of the 16 patients (25%) with ACM etiologies and 18 of the 27 patients (67%) with NACM etiologies. Nineteen of the 22 transplanted patients (3 ACM and 16 NACM) survived to discharge (Table 2). Among the transplanted patients who died, only one in the NACM group had received BAL support.
Table 3 compares the sensitivity, specificity, the positive and negative predictive values and the predictive accuracy of the London and Clichy prognostic scoring systems at the time of admission. In the ACM group, the sensitivity (the proportion of patients fulfilling the criteria who died) was superior for the Clichy criteria (86% vs. 71%, respectively), while the specificity (the proportion of patients surviving who did not fulfill criteria) was better for the London criteria (78% vs. 56%, respectively). The PPV, NPV and PA of both criteria were low and of marginal benefit in assessing the prognosis.
Analysis of the specific elements of the London criteria for the ACM hepatotoxicity (Table 4) showed that the PPV and PA of metabolic acidosis with an arterial pH <7.30 were 100% and 87%, respectively. In contrast, the PPV and PA for the combination of all 3 variables in the ACM group was 0% and 47%, respectively. The NPVs for either an arterial pH <7.30 or all 3 variables were 78% and 47%, respectively.
For the patients with NACM etiologies of FHF (Table 3), the overall London criteria were superior to those of the Clichy criteria: sensitivity (96% vs 80%, respectively), specificity (100% vs 100%, respectively), PPV (100% vs 100%, respectively), NPV 67% vs 29%, respectively) and PA (96% vs 82%, respectively). As shown in Table 4, which assesses the individual scored elements of the London criteria, the PPV and PA of a PT INR of >6.5 were 100% and 57%, respectively. In contrast, the presence of =3 of the 5 prognostic variables had a similar PPV (100%), but a higher PA (93%) for the need for LT in our patients. However, the NPV for a PT INR >6.5 or =3 of the 5 prognostic variables in the NACM group were only 14% and 50%, respectively.
DISCUSSION
Distinguishing the patients with FHF who will require urgent LT from those who will likely to recover with medical therapy alone remains problematic. Recognizing the subset of patients who require LT is critically important because the rapid progression of FHF requires immediate treatment since death or the development of absolute contraindications for LT often occurs before a donor organ becomes available. In view of the severe shortage of donor organs and the previous reports that up to 60% of patients with FHF due to etiologies such as ACM and viral hepatitis may recover without LT9, 21-24), it is also imperative to avoid unnecessary LT for the patients who will recover with receiving medical therapy alone. The urgency of establishing an accurate prognosis is accentuated by the fact that FHF patients in the U.S. are often cared for at hospitals for several days before being transferred to a transplant center11, 16). The severity of FHF at the time of admission to our center may be partly explained by the median 4.4 days before transfer, which is similar to the median of 4 days reported by the University of Pittsburgh16).
The London and Clichy prognostic criteria are the most frequently used systems to determine the probability of survival without LT for patients with FHF. The London criteria were developed by retrospectively identifying the most discriminatory combination of prognostic variables via stepwise logistic regression analysis of 588 patients who suffered with ALF and who were treated medically between 1973 and 198510). The accuracy of the London criteria was further assessed by retrospective analysis of another 175 patients (121 ACM and 54 NACM etiologies) who were treated during 1986-87. The specific criteria for the ACM and NACM etiologies were selected based on the probability of survival (5%) with receiving medical therapy alone. Although the grade of hepatic encephalopathy was a prognostic indicator for FHF due to ACM etiologies, it was not for the NACM etiologies. The Clichy prognostic criteria were validated in a series of 90 patients who suffered with with FHF due to acute viral hepatitis (primarily hepatitis B) and who had factor V activities <50% of normal12). Among the 43 patients with stage III-IV hepatic encephalopathy who met these criteria, factor V activities <20% for the patients <30 years of age or <30% for the patients >30 years of age were associated with survivals of 10% with receiving medical therapy alone and 84% with LT. The PPV and NPV for death with medical therapy alone were 82% and 98%, respectively. Application of these criteria identified 95% of the survivors. Currently, the Clichy prognostic criteria are exclusively used by some centers for all patients with FHF, regardless of the etiology15).
The London and Clichy prognostic criteria have not been previously compared in American patients with FHF that's due to both the ACM and NACM etiologies. Several sources of potential bias must be considered when comparing our results with those of the other previous reports. Although all 43 patients received standard intensive care from a multidisciplinary team, they were also eligible for experimental BAL support under the protocol18, 19). Indeed, 8 patients with ACM etiologies and 10 with NACM etiologies underwent BAL support (Table 1). Comparing the prognostic scoring systems only at the time of admission, rather than serially, might have theoretically underestimated the prognostic power of either system. Although we intended to enroll consecutive patients admitted with FHF, it was necessary to exclude those patients who had received fresh frozen plasma before admission or they only had a frozen blood specimen that would have underestimated the factor V activity. Despite this potential selection bias, the etiologies of FHF in our 43 patients and the proportions of patients with specific FHF etiologies were quite similar to those reported for 295 consecutive American patients with FHF from 13 U.S. centers25). Inclusion of patients undergoing transplantation (25% of the ACM group and 67% of the NACM group, respectively) as true positives also introduces a potential bias that's common to many reported series15, 16), since it cannot be proved that every patient undergoing LT would have died with receiving only continued medical therapy. In this regard, it is important to note that every patient who died during medical therapy met the London and/or Clichy criteria for a survival prognosis of =5% without LT.
For our 16 patients with FHF due to ACM toxicity (Table 3), theeir overall PPV and PA of the London criteria (71% and 75%, respectively) were superior to those of the Clichy criteria (60% and 69%, respectively). Although the PPV (the proportion of patients fulfilling criteria who died) of the London criteria (71%) was greater than that of the Clichy criteria (60%), neither the PPV nor the NPV (the proportion of patients not fulfilling the criteria who survived) of the London criteria were as high as those of the several previously published studies9, 27-29). The PPV (71%) and PA (75%) of the London criteria of our patients were inferior to the PPV of 84% and the PA of 85% as originally reported by O'Grady et al.10), but they were in close agreement with the PPV of 73% and the PA of 72% as reported by Anand et al. for 145 patients who were assessed in the U.K. between 1990-9429). In contrast, our sensitivity, specificity, PPV and PA were comparable or greater than the values reported by Izumi et al.31) for 81 patients with ACM-induced FHF. Specifically, the PPV of the Clichy criteria in our study (60%) was in close agreement with the 57% reported by Izumi et al.30) for 81 ACM patients who were not transplanted and they had a factor V activity of <10%, regardless of the grade of hepatic encephalopathy. The PPV of 49% reported by Izumi et al.30) for factor V levels <20% was lower than our PPV with using age-adjusted criteria. Although their PPV of a factor V level <20% for the subgroup of ACM patients with stage III-IV hepatic encephalopathy increased to 73%, it remained inferior to the PPV of 92% for the London criteria. However, it is important to note that among the 110 FHF patients reported on by Izumi et al (88 ACM etiologies and 22 NACM etiologies) the admission levels of factor V were significantly lower in the 49 patients who died (median: 5%, range: 1~27%) than in the 61 patients who survived (median: 10%, range: 2~70%)30).
The most accurate predictor of death without LT among our ACM patients was an arterial pH <7.30 on admission. Whereas an arterial pH <7.30 had a high PPV, NPV and PA in both our study and the study of O'Grady et al.10), Anand et al.29) and Shakil et al.16) reported much lower values.
Conversely, the PPV, NPV and PA of the other 3 variables for our patients were substantially lower than the values reported in the three other previous series10, 16, 29). A major confounding factor is the likelihood that BAL support contributed to the survival of more patients with ACM toxicity in our series than was predicted by the London criteria. Grade III or IV encephalopathy is more common in the non-ACM group (85%) than that in the ACM group (50%), and this may be a risk factor for a poor outcome in those groups31), yet BAL is known as a more effective treatment for hepatic encephalopathy than for other impaired hepatic functions32, 33). This speculation is supported by the results of a randomized, controlled trial of BAL for treating FHF that's due to ACM toxicity, and the results showed a significantly increased survival compared to standard intensive care32). Demetriau et al. reported that ALF patients with known etiologies such as virus, ACM, other drugs or chemical toxicities have a better prognosis, and ACM-FHF can be associated with a high spontaneous recovery rate; however, a few patients suffering withsevere variants of ACM-FHF can rapidly progress to cerebral edema and death33). Further studies with a large number of patients are needed to identify these additional prognostic variables.
In our 27 patients with NACM etiologies for FHF, the overall PPV of both the London and Clichy prognostic criteria was 100%, while the PA was 96% and 82%, respectively (Table 3). Our results with the Clichy criteria compared favorably to the prospective results at admission to the hospital of Izumi et al.30) who reported a PPV of 85% for a factor V level <20% and 100% for a factor V level <10% in 17 of 22 NACM patients with grade I-IV hepatic encephalopathy and who were not transplanted. For those patients with grade III or IV encephalopathy, the PPV was 91% for a factor V level <20%. The overall PPV and PA of the London criteria for the 17 patients reported by Izumi et al.30) were 93% and 88%, respectively, compared to 100% and 96%, respectively, for our patients. Pauwels et al. retrospectively compared both prognostic scoring systems for 81 French adults with NACM FHF and who were treated with medical therapy alone on admission and then again at 48, 24 and <24 h before death24). The mortality with medical therapy alone was 81%. On admission, the PPV of the London and Clichy criteria was 96% and 90%, respectively, while the PA was 80% vs. 60% respectively. The PPV did not increase when it was reassessed 48, 24 or <24 h before death for either London or Clichy criteria, but the PA predictably increased as death became imminent. In contrast to our results and those of others for the PPVs and PAs of the NACM etiologies of FHF, Anand et al. reported an overall PPV and PA of only 68% and 61%, respectively, for 145 patients who were treated in the U.K.29). Unfortunately, the overall PPV was not reported by Shakil et al.16), although they found acceptable PPVs and PAs for the individual components of the London criteria in 144 American patients who were treated for FHF due to NACM etiologies; these patients were treated between 1982-95 at the University of Pittsburgh. The results in non-transplanted patients with FHF reported by Izumi et al.30) and Pauwels et al.24) indicate that the potential bias of including transplanted patients as true positives in our study may have caused only a modest increase in the PPV and PA.
While our PPV of a PT INR >6.5 was comparable to those of all the other previous reports10, 16, 29), the PPV and PA of =3 of the 5 prognostic variables in our study were similar to those of O'Grady et al.10) but they were appreciably higher than those of Anand et al.29) and Shakil et al.16) The overall PPV (100%) and PA (96%) for the combination of the two scored elements were similar to the results of O'Grady et al.10) but they exceeded those reported by others24-29). Our PPV and PA of the Clichy criteria for patients with NACM etiologies were 100% and 82%, respectively. These results were slightly greater than those previously reported by others24-30).
Our overall NPV was 67% for the London criteria for patients suffering with the NACM etiologies of FHF, while the values reported by others ranged from 25% to 82%. In contrast, the NPV for the Clichy criteria was only 29%, which was nearly identical to the NPV of 28% on admission in the study of Pauwels et al. on non-transplanted patients24). The fact that our PPVs were greater than the sensitivities (the proportion of fatalities meeting the criteria), while our specificities (the proportion of survivors not meeting the prognostic criteria) were much greater than our NPVs (Table 3), indicates there was excessive mortality for the patients suffering with NACM FHF and who did not fulfill the criteria at the time of admission for death without LT.
To conclude,, overall, the London criteria more accurately predicted the need for transplantation, but neither the London nor Clichy prognostic criteria accurately predicted the outcome of our patients with FHF due to ACM. The positive impact of BAL support on our ACM patients may have contributed to the survival without LT, which exceeded the predictions of both prognostic systems33). In contrast, both the London and Clichy prognostic criteria exhibited high PPVs for survival, but the PA of the London criteria was superior. Additional multicenter studies should be conducted to refine these prognostic scoring systems so physicians can rapidly identify the patients suffering with either the ACM or NACM etiologies of FHF, and who will survive without undergoing liver transplantation.
Notes
This study was supported, in part, by a Glaxo Wellcome Grant from the Korean Association for the Study of Liver Disease, and the grant was awarded to Won-Choong Choi, M.D.
Abbreviations
FHF
fulminant hepatic failure
LT
liver transplantation
ACM
acetaminophen
NACM
non-acetaminophen
PPV
positive predictive value
NPV
negative predictive value
PA
predictive accuracy