Agenesis of the Dorsal Pancreas: A Case Report and Review of the Literature
Article information
Abstract
Partial or complete agenesis of the dorsal pancreas is a rare congenital anomaly that results from the embryological failure of the dorsal pancreatic bud to form the body and tail of the pancreas. To date, four cases have been reported in Korea. We report an additional case; a 25-year-old woman presented with diabetes mellitus and abdominal pain. Abdominal computed tomography (CT) revealed a normal-appearing pancreatic head, but the body and tail were not visualized. Endoscopic cholangiopancreatogram (ERCP) revealed a short pancreatic duct in the uncinate process and the head and the duct of Santorini draining into the minor papilla. Abdominal magnetic resonance imaging (MRI) findings were similar to the CT and ERCP results. The patient was diagnosed with partial agenesis of the dorsal pancreas by CT, ERCP and MRI.
INTRODUCTION
The pancreas develops from dorsal and ventral buds that arise from the caudal region of the embryonic foregut1). The ventral bud gives rise to the lower portion of the pancreas head and the uncinate process, while the dorsal bud elongates to form the upper head, body and tail1). Partial or complete agenesis of the dorsal pancreas is a rare congenital anomaly that results from embryologic failure of dorsal pancreatic budding in the developing fetus1-3). To date, four cases have been recorded in Korea4-7). Here we report an additional case in a 25-year-old woman with partial agenesis of the dorsal pancreas, and review the medical literature.
CASE REPORT
A 25-year-old woman was admitted to Chonnam National University Hospital with a four-week history of intermittent abdominal pain. She had an 11-year history of insulin-dependent diabetes mellitus. There was no previous history of peptic ulcer diseases, hepatobiliary disease, cholecystitis with gallstones or pancreatitis. On admission, her abdomen was soft and not distended, but was tender to deep palpation in the epigastric region. Physical examination was otherwise within normal limits. Laboratory evaluation revealed a white blood cell count of 5,700/mm3 (normal: 6,000-10,000), hemoglobin 11.0 g/dL (normal: 12-16), platelet count 285,000/mm3 (normal: 130,000-450,000), serum albumin 3.5 g/dL (normal: 3.0-5.0), aspartate aminotransferase 32 U/L (normal: 5-37), alanine aminotransferase 43 U/L (normal: 5-40), alkaline phosphatase 431 U/L (normal: 39-117), and γ-glutamyl transpeptidase 524 U/L (normal: 7-49). The total bilirubin was 0.38 mg/dL with 0.06 mg/dL direct fraction (normal: 0.2-1.2/0.05-0.3). Serum amylase and lipase were within normal range. An abdominal computed tomography (CT) scan showed a normal-appearing pancreatic head and complete absence of the body and tail (Figure 1A, B). Endoscopic retrograde cholangiopancreatogram (ERCP), opacified from the major papilla, showed a short duct in the uncinate process and head and the duct of Santorini draining into the minor papilla (Figure 2). Abdominal magnetic resonance imaging (MRI) showed a pancreatic head, but the body and tail were not visualized (Figure 3). These findings were similar to those of the CT and ERCP. The combined CT, ERCP and MRI findings were considered diagnostic of a partial agenesis of the dorsal pancreas. After symptomatic treatment, she was discharged and followed regularly.
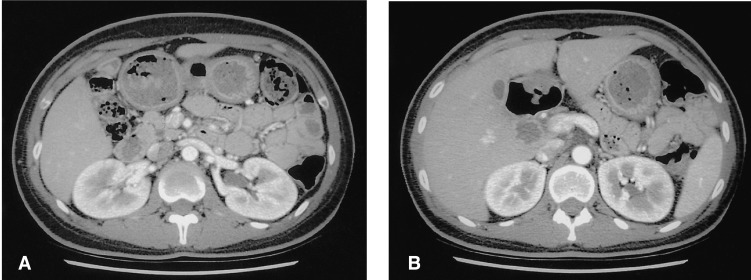
Abdominal computed tomography (CT) reveals a normal-appearing pancreatic head (A) and complete absence of the body and tail (B).

Endoscopic cholangiopancreatography (ERCP) shows a short duct in the uncinate process and head; the duct of Santorini drains into the minor papilla.
DISCUSSION
The human pancreas develops from the ventral and dorsal buds of the foregut endoderm1). The ventral bud forms the uncinate process and the posteroinferior part of the head. The Wirsung duct drains along with the bile duct through the major papilla1). The dorsal bud forms the remaining ventrosuperior part of the head, the isthmus, the body and the tail of the pancreas and drains through the Santorini duct into the minor papilla1).
Agenesis of the dorsal pancreas is derived embryologically from the absence or regression of the dorsal bud1-3). This anomaly may be partial or complete. In partial agenesis of the dorsal pancreas, the minor papilla, duct of Santorini or the pancreatic body are present. In complete agenesis of the dorsal pancreas, the neck, the body and the tail of the pancreas, duct of Santorini and minor papilla are absent8). Few cases of agenesis of the dorsal pancreas have been reported in the English literature2, 3). Most reports describe a single case presenting with diabetes mellitus, weight loss, pancreatitis, jaundice and duodenal obstruction8-12).
Only five cases of agenesis of the dorsal pancreas (including the present case) have been reported in Korea (Table 1). The patients were 36 weeks to 66 years of age and included three men and two women. Among the five cases, one was reported as a stillborn fetus at autopsy4). There were two complete and three partial types reported.
In review of the English literature, diabetes mellitus has been noted in most cases with this anomaly. Also, in the Korean cases including ours, three of the four adult cases, had diabetes mellitus. Because the body and tail of the pancreas have most of islet cells, the absence of the body and tail, with this anomaly, contributes to the development of diabetes mellitus13). However, evidence of diabetes mellitus in previous reported cases has been inconsistent. Scattered islets of Langerhans with destruction of glandular parenchyma, in pancreatic head tissue, were found microscopically in the case reported by Fukuoka et al2).
Agenesis of the dorsal pancreas has been most frequently identified from imaging studies during investigation of abdominal pain. The abdominal pain has been assumed to be due to pancreatitis, duodenal obstruction, autonomic neuropathy, or sphincter of oddi dysfunction8-12). In the Korean cases including ours, three patients had abdominal pain. Among the three cases with abdominal pain, one had a common bile duct stone with pancreatitis, the cause of abdominal pain in other two cases remained undetermined.
Wildling et al and Schnedl et al reported familial occurrence of agenesis of dorsal pancreas in the mother and her sons14, 15). In these reports, the authors suggested that the genetic mode of transmission for this anomaly is most likely autosomal dominant or X-linked dominant. However, in the Korean cases including ours, there was no family history of the anomaly.
Agenesis of the dorsal pancreas is usually suggested on abdominal ultrasonogram (US), CT, or MRI when the body and tail of pancreas are not visualized ventral to the splenic vein15-21). When agenesis of the dorsal pancreas is suggested by imaging studies, diagnostic possibilities to exclude fat replacement of the pancreas and atrophy following pancreatitis should be considered. In fat replacement of pancreas, the entire gland is usually involved and the pancreatic duct is present, whereas with atrophy following pancreatitis a relevant clinical history usually exists18, 20, 22). However, imaging studies such as US, CT and MRI and a relevant clinical history are not sufficient to establish the diagnosis of agenesis of the dorsal pancreas. ERCP is necessary to confirm agenesis of the dorsal pancreas because it is important to define the anatomy of the pancreatic ducts when differentiating this anomaly from other diagnostic possibilities such as pancreatic divisum and pancreatic neoplasm15, 18, 21). However, ERCP is invasive procedure and operator-dependent for successful identification of opacity of the main and accessory pancreatic duct. By contrast, MR cholangiopancreatogram (MRCP) clearly demonstrates pancreatic duct morphology15, 18, 21). In cases where cannulating the pancreatic duct fails, MRCP may be helpful. Therefore, the combined use of CT and ERCP or MRCP is useful for confirmation of the diagnosis of agenesis of dorsal pancreas15, 18, 21). In the reported Korean cases, the diagnosis was suspected by CT, but an ERCP was required for confirmation. Only one case underwent a laparotomy for confirmation due to the possibility of a pancreatic neoplasm6).
In Korea, agenesis of dorsal pancreas is a very rare congenital anomaly that may be associated with diabetes mellitus and abdominal pain. However, hereditary mechanisms may play a role in the development of this anomaly but remains to be further clarified. If agenesis of the dorsal pancreas is suspected, the combined use of CT and ERCP or MRCP is needed for confirmation of the diagnosis.