A Case of Primary Intestinal Lymphangiectasia
Article information
Abstract
A 26-year-old male patient who had an 8 years history of recurrent peripheral edema with diarrhea and hypoproteinemia was evaluated. Endoscopic jejunal and ileal biopsy revealed markedly dilated mucosal lymph vessels with no evidence of inflammation. 99mTc-labeled human serum albumin (HSA) scintigraphy showed significant activity accumulating in the gastrointestinal tract to represent 99mTc-HSA leakage into the bowel lumen. A diagnosis of protein losing enteropathy and intestinal lymphangiectasia could be made. After treatment with a high protein and fat restricted diet, his symptoms subsided and the serum protein level was normalized.
INTRODUCTION
Primary intestinal lymphangiectasia (PIL) is a well recognized disease characterized by markedly dilated intestinal lymphatics, hypoproteinemia, generalized edema and lymphocytopenia1). The excessive loss of protein into the gastrointestinal tract is a major factor for the development of hypoproteinemia in these patients and its demonstration is important in the diagnosis of intestinal lymphangiectasia1–10).
Various methods, including α1-antitrypsin intestinal clearance test and tests using radiolabeled macromolecules, have been used to detect gastrointestinal protein losses1,2). Recently, 99mTc-human serum albumin (HSA) abdominal scintigraphy, which is a simple, safe and readily available method to detect protein loss into bowel lumen, was introduced and proved to be useful to screen protein losing enteropathies5–7).
We experienced a case of primary intestinal lymphangiectasia in a patient whose enteric protein loss was detected by 99mTc-HSA abdominal scintigraphy, and that was confirmed by endoscopic intestinal biopsy showing markedly dilated intestinal lymphatics.
CASE REPORT
A 27-year-old male was admitted to Yeungnam University Hospital in December of 1991 because of diarrhea and aggravated edema of the lower extremities over 8 months.
8 years prior to admission, he suffered from an edema of the lower extremities and abdominal distension, but the symptoms subsided spontaneously without any special treatment and no further investigations were done. From 4 years before admission, he had intermittently experienced an edema of the lower extremities which was always associated with diarrhea. He was diagnosed as having hypoalbuminemia by a local physician and was referred to this hospital for gastrointestinal evaluations.
He was a non-smoker, non-alcoholic and had no significant past medical history. His family history was unremarkable and he had no known allergy. There was no history of fever, vomiting, abdominal pain, weight loss or urinary problems.
On admission, he was afebrile and relatively healthy, and vital signs were stable. Physical examination revealed severe pitting edema on both lower extremities. Heart, lungs and abdomen were normal, and lymph nodes were not palpated.
Peripheral blood hemoglobin was 15.5gm/dl, white blood cell count 5.4×109/L with 74% neutrophiles and 15% lymphocytes, platelet count 297×109/L and ESR was 25 mm/hour. Serum sodium, potassium, chloride, magnesium, urea, nitrogen, creatinine, AST, ALT, alkaline phosphatase, transferrin and plasma fibrinogen were all normal. Skin tests to PPD, ANA, LE cell and RA factor were negative. Urinalysis was normal. Serum total cholesterol was 102 mg/dl, calcium 7.6 mg/dl. total serum protein 3.9gm/dl, albumin 1.8gm/dl, α1-globulin 0.3 gm/dl, α2-globulin 0.53 gm/dl, β-globulin 0.64 gm/dl and γ-globulin was 0.62 gm/dl. Serum immunoglobulin (Ig) G was 412.0 mg/dl, IgA 89.9 mg/dl, IgM 113.0 mg/dl, IgD 2.4 mg/dl and IgE was 126.0 IU/ml.
The chest X-ray and barium enema showed no abnormal findings. The small bowel series revealed irregular segmental luminal narrowing in jejunum and ileum(Fig. 1). Small intestinal endoscopic and colonoscopic study showed the presence of whitish granular spots scattered throughout the mucosa of the jejunum and terminal ileum(Fig. 2), but the esophagus, stomach, duodenum and colon showed normal findings. Biopsy specimens taken from jejunum and terminal ileum revealed markedly dilated submucosal lymphatics with no evidence of inflammation (Fig. 3).
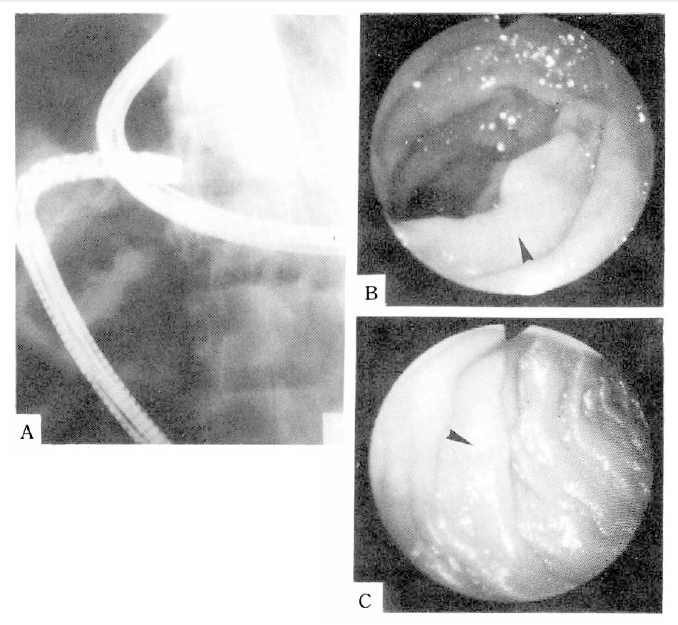
Small intestinal enteroscopy performing under fluoroscopic guidance (A) and endoscopic findings showing multiple whitish granular spots scattered throughout the mucosa of the jejunum (B) and terminal ileum (C).

Microscopic section reveals markedly dilated submucosal lymphatics without inflammation (arrow). H & E stain (×1000)
For the demonstration of protein loss from intestinal mucosa, 99mTechnetium-HSA scintigraphy was performed: 20 mCi of 99mTc-HSA was injected intravenously and then abdominal radioactivity was counted serially. The activity was apparent in the right lower quadrant and transverse colon at 6 hours, and in the descending and sigmoid colon at 24 hours after injection, indicating a significant loss of serum protein into bowel lumen in this patient (Fig. 4).

99mTc-HSA Scintigraphy: The radioactivity is noted in the heart, great vessels, liver and spleen at 30 minutes (A), in the right lower quadrant and transverse colon at 6 hours (B) and in the transverse and sigmoid colon at 24 hours (C) after intravenous injection of 99mTc-HSA.
Under the diagnosis of PIL, the patient received a high-protein and fat-restricted diet. Immediately after starting these dietary treatments, his edema and diarrhea subsided, and 4 weeks later the serum albumin level was normalized.
DISCUSSION
Since Waldmann et al’s first description in 1961, intestinal lymphangiectasia has been recognized as a distinct disease entity characterized by hypoproteinemic edema, diarrhea, steatorrhea, lymphocytopenia and enteric loss of protein resulting from the markedly dilated lymphatic channels of the small intestine1,8,10,12).
It may appear as a primary form caused by congenital malformation of generalized lymphatic systems or secondary form due to various conditions including inflammatory disease, cardiac disease, Whipple’s disease, sarcoidosis, tuberculosis, retroperitoneal fibrosis, lymphoma etc1,8,10,11,12).
PIL is a disease primarily affecting children and young adults, usually occurring from birth to 30 years of age in over 90% of the cases, and both sexes are affected equally1,3,4,9). From the review of previously reported cases, the initial manifestation of intestinal lymphangiectasia is usually hypoproteinemic edema and diarrhea1,8,9,13,14). In some patients, vague abdominal discomfort or steatorrhea can be noted and, in cases of severe enteric protein loss, intestinal obstruction, chylous ascites, hypocalcemic tetany or growth retardation may develop10).
Secondary intestinal lymphangiectasia is usually adult-onset and may have certain distinguishing features from PIL: elevated ESR, normal or increased immunoglobulin levels, positive ANA or LE test and other evidence of inflammatory processes11). In our cases, considering several features such as the early onset of symptoms, namely negative ANA and LE test, near normal level of ESR, decreased IgG level and absence of other evidence of inflammation, PIL was more compatible than secondary types.
The small bowel series are abnormal in over 2/3 of the cases and common findings are thickening and enlargement of the valvulae coniventes of the jejunum and ileum and distal dilution of barium, indicating hypersecretion and slight dilatation of the lumen14). Pedal lymphangiograms are abnormal in almost all cases and the usual features are hypoplasia of the lymphatic systems of the lower extremities, nonvisualization of the thoracic duct and agenesis of the retroperitoneal lymph nodes15).
Demonstration of gastrointestinal protein loss as a cause of hypoproteinemia, which can differentiate from other causes of hypoproteinemia, is essential to make the diagnosis of the intestinal lymphangiectasia10). Methods evaluating enteric protein loss include quantitation of protein loss by determining fecal clearance of α1-antitrypsin or measurement of the magnitude of protein loss by counting fecal excretion of radioactivity following intravenous administration of the radiolabeled macromolecules such as 125I-serum protein, 51Cr-serum proteins, 59Fe-iron dextran, 95Nb-albumin, 67Cu-ceruloplasmin, or 131I-PVP. Recently 99mTc-human serum albumin (HSA) abdominal scintigraphy was introduced for this purpose and compared with other methods, 99mTc-HSA scintigraphy had a lower radiation burden on the patient and was more readily available, relatively inexpensive and easily performed5,6,7). In our cases, we performed 99mTc-HSA abdominal scintigraphy to evaluate the cause of hypoproteinemia and successfully demonstrated that significant enteric protein loss was present.
Endoscopic findings of PIL are numerous whitish spots over the mucosa, white villi and chyle-like material covering the mucosa in the jejunum and terminal ileum, which become more prominent after ingestion of a high-fat meal the night before the procedure16). The biopsy from these sites represents markedly dilated lymph vessels of the mucosa and submucosa, the hallmark lesion of the disease17). Diagnosis of PIL can be confirmed by these characteristic endoscopic and histological findings. In our cases, endoscopic study of jejunum and terminal ileum showed multiple whitish granular spots on the mucosa and a biopsy of these lesions revealed characteristic histological findings.
There are no specific treatments for patients with PIL10). Segmental resection may be recommended in cases involving localized segment of the intestine and, in some cases, marked improvement with antiplasmin therapy was reported18,19). But, in over half of the cases, treatment with a high-protein, fat-free diet, and supplemetation of medium-chain triglyceride that is absorbed directly into the portal system without developement of lacteal engorgement, is usually effective in preventing and alleviating the symptoms20). In our cases, diarrhea and edema all subsided immediately after dietary treatment alone, and the serum albumin level was also normalized.
In conclusion, we are presenting a case of PIL in which the diagnosis was confirmed by 99mTc-HSA scintigraphy and characteristic intestinal endoscopic and histological findings. Because of the relatively good response to the diet therapy, the early diagnosis of PIL is very important for the management of these patients. Considering the major role of gastrointestinal protein loss in the pathogenesis of the intestinal lymphangiectasia, it is important to detect the presence of enteric protein loss using various non-invasive methods such as 99mTc-HSA scintigraphy in any suspected cases.
