Twenty-Four Hour Urinary C-peptide and Fasting Plasma C-peptide as Indicators of Metabolic Control in 83 Insulin Dependent Diabetic Patients
Article information
Abstract
In order to evaluate the usefulness of 24-hour urinary C-peptide and fasting plasma C-peptide as metabolic index, C-peptide was measured in 24-hour urine and in fasting plasma of 83 insulin dependent diabetic patients. Using multiple linear regression, HbAlc was analyzed as a dependent variable versus independent variables such as daily insulin dose, fasting plasma C-peptide and 24-hour urinary C-peptide. Twentyfour hour urinary C-peptide was significantly correlated with fasting plasma C-peptide showed significant inverse correlations with the daily insulin dose. But neither 24-hour urinary C-peptide nor fasting plasma C-peptide was significantly correlated with HbAlc as a dependent variable. These results show that there was no difference of usefulness between 24-hour urinary C-peptide and fasting plasma C-peptide in relation to HbAlc and daily insulin dose in insulin dependent diabetic patients.
INTRODUCTION
The measurement of plasma C-peptide can be used to determine B-cell secretory function1,2). Meistas et al.3) demonstrated that there was a highly significant correlation between 24-h urinary C-peptide excretion and insulin secretion in 50 normal subjects. Aurbach-Klipper et al.2) found a highly significant correlation between 24-h urinary C-peptide and fasting plasma C-peptide in 27 type 1 diabetic children. It was observed that C-peptide clearance was independent of creatinine clearance over a wide range1). Previous studies4,5) have indicated that 24-h urinary C-peptide excretion is best expressed in relation to 24-h urinary creatinine.
The inverse relationship between 24-h urinary C-peptide and daily insulin dose in insulin dependent diabetic patients was reported4,6–8). Sjöberg et al.8), in addition, published that HbAlc had an inverse correlation with 24-h urinary C-peptide but no correlation with fasting plasma C-peptide in 28 type 1 diabetics with onset before the age of 30. Dahlquist et al.9) showed that fasting plasma C-peptide level had an inverse relation to both HbAlc concentration and daily insulin dose in a prospective study of 131 diabetic children. There is no unanimous conclusion regarding the influence of residual B-cell function as expressed by fasting plasma C-peptide or 24-h urinary C-peptide on metabolic control as measured by daily insulin dose or HbAlc.
Sjöberg et al.8), with the above-mentioned results, proposed that a more sensitive method for demonstrating B-cell secretory function would seem to be 24-h urinary C-peptide in type 1 diabetes mellitus. But a study to evaluate the difference between a 24-h urinary or fasting plasma C-peptide in respect to metabolic control as measured by HbAlc or daily insulin dose had not been attempted.
This study was designed to investigate which the 24-h urinary or fasting plasma C-peptide is the better indicator or B-cell secretory function, in connection with metabolic control measured as HbAlc and daily insulin dose, in insulin-treated diabetic patients.
SUBJECT AND METHODS
1. Subjects
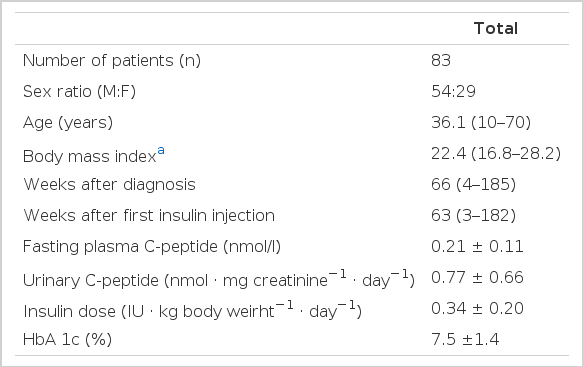
The subjects included 83 insulin dependent diabetes mellitus patients who were admitted to Steno Memorial Hospital from June 1982 to December 1985, The diagnosis of insulin dependent diabetes was based upon clinical appearance and made by a physician either at this Hospital or at the Department of Internal Medicine no more than four weeks before admission to Steno Memorial Hospital
Clinical details are shown in Table 1.
Patients were excluded if they had other endocrine or chronic diseases.
2. Methods
The physicians were aware of this prospective study, but criteria or directions for insulin treatment were neither discussed or given.
The actual insulin dose/kg body weight during the previous 24-h was recorded.
Blood samples were collected after an overnight fast for fasting plasma C-peptide and HbAlc. Urinary samples for the preceding 24-h were collected.
Plasma C-peptide and urinary C-peptide concentrations were measured by radioimmunoassay employing antibody M123010).
Twenty-four hour urinary C-peptide excretion was expressed in relation to 24-h urinary creatinine (nmol · mg creatinine−1 · day−1)4,5). HbAlc was determined as previously described11).
3. Statistical Analysis
The correlation between fasting plasma C-peptide and 24-h urinary C-peptide was analyzed by linear regression.
Multiple linear regression analysis was performed on an HP-85 computer, using the general statistics package for multiple linear regression12,13). The variables were arranged into two groups as follows: the first included HbAlc · fasting plasma C-peptide · daily insulin dose, and the other HbAlc · 24-h urinary C-peptide · daily insulin dose. Multiple linear regression analysis was done for HbAlc as a dependent variable versus independent variables such as fasting plasma C-peptide (or 24-h urinary C-peptide) and daily insulin dose. The significance was judged by the F test.
Data are expressed as mean±SD unless otherwise stated.
The significance level of tests was taken as p< 0.05.
RESULTS
Fig. 1 shows that there was a significant correlation between 24-h urinary C-peptide (nmol · mg · creatinine−1 · day−1) and fasting plasma C-peptide (nmol/l) in 83 patients (r = 0.64; p<0.001).
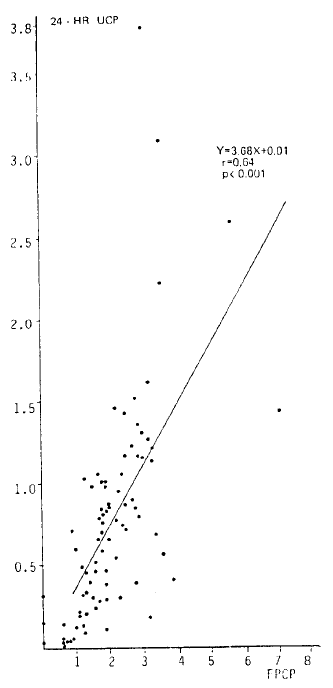
Relation between fasting plasma C-peptide (nmol/l) and 24-h urinary C-peptide (nmol · mg creatinine−1 · day−1) in 83 insulin-treated diabetic patients.
Table 2 shows that both fasting plasma C-peptide and 24-h urinary C-peptide were inversely correlated with the daily insulin dose (r = −0.473; p<0.001, r = −0.398; p<0.001, respectively). Neither fasting plasma C-peptide nor 24-h urinary C-peptide was significantly correlated with HbAlc (r = −0.157; p>0.05, r = −0.157; p>0.05, respectively). Daily insulin dose had no relation to HbAlc (r = 0.068; p>0.05).
Multiple correlation coefficents (R) were 0.226 among HbAlc. fasting plasma C-peptide · dally insulin dose, and 0.212 among HbAlc. 24-h urinary C-peptide · daily insulin dose. Neither group exhibited significant multiple correlations (p>0.05, in both).
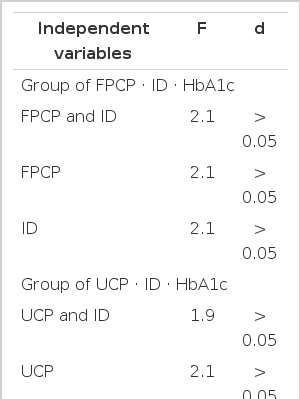
To examine the influence of independent variables on HbAlc as a dependent variable, the analysis of variance was performed. Table 3 shows that HbAlc was not influenced by each independent variable or paired independent variables in both groups (p>0.05, all). This result became more pronounced by the calculation of multiple linear regression equations and regression coefficients in each group. In the group of HbAlc · fasting plasma C-peptide · daily insulin dose, multiple linear regression was
HbAlc = 8.565–3.075 · fasting plasma C-peptide −1.310 · daily insulin dose. None of the two regression coefficients reached the level of statistical significance (−3.075, F = 3.893; p>0.05, −1.310, F = 2.202; p>0.05). In the other group, the equation was
HbAlc = 8.230–0.477. 24-h urinary C-peptide-1.018. daily insulin dose. Neither of the two regression coefficients reached the level of statistical significance (−0.477, F = 3.375; p>0.05, −1.108, F = 1.698; p>0.05).
DISCUSSION
We found that 24-h urinary C-peptide was significantly correlated with fasting plasma C-peptide in agreement with previous studies3,4).
In the present study, both 24-h urinary C-peptide and fasting plasma C-peptide showed significant inverse correlations with the daily insulin dose. This finding is consistent with several other reports4,6–9).
But neither 24-h urinary C-peptide nor fasting plasma C-peptide was significantly correlated with HbAlc. Sjöberg et al.8) found that 24-h urinary C-peptide was inversely correlated with HbAlc. Fasting plasma C-peptide did not show statistically significant correlation with HbAlc but the P value was 0.05 < p < 0.10. The study of Sjöberg et al. was done in 28 type 1 diabetics with onset before the age of 30 and duration of diabetes between 1 and 6 years. The present study was done in 83 insulin denendent diabetic patients with a wide range of (10–70 years) and one week after admission although diagnosis was between 4 weeks and 185 weeks, so the variation in B-cell secretory function was probably larger both due to the inculsion of older patients14) and the fact that some were just in the remission phase14).
Dahlquist et al.9) found a week but statistically significant inverse relation between fasting plasma C-peptide and HbAlc (r = −0.25; p < 0.01). In the multicenter study by Dahlquist et ai., the patients were selected so that none had had their insulin regimens changed during the past 2 months. They could have been investigated at a time point when B-cell secretory function was decreasing before changes in HbA1c.
We also found no influence of fasting plasma C-peptide or 24-h urinary C-peptide and daily insulin dose as independent variables on HbAlc as a dependent variable. Several factors probably contribute to this finding. Firstly, HbAlc level may not be helpful in deciding acute manipulations of insulin therapy15). A specific HbAlc level may not indicate the timing and the magnitude of change of insulin regimen, especially at the time when the diabetic patient enters remission. Secondly, endogenous insulin secretion is only one of many factors that influence metabolic control in diabetic patients16). Other influencing factors are peripheral insulin resistance, adherence to the prescribed diet, psychological problems, physical stress, and exercise17).
Exogenous insulin would also obscure the significance of residual B-cell secretory function. Thirdly, the reproducibilities of 24-h urinary C-peptide and fasting plasma C-peptide as B-cell secretory function tests should be considered. The reproducibilities of these two tests have not been evaluated in insulin dependent diabetic patients. Though they studied non-insulin dependent diabetic patients, Takai et al.18) found that the consecutive measurement of 24-h urinary C-peptide (ug/day) revealed a considerable day-to-day variation in 17 patients (the average coefficient of variation = 39.1%). They suggested that the variability could be ascribed to the change in diet, the effect of exercise, and the variable urinary clearance of C-peptide.
In conclusion, 24-h urinary C-peptide was no more advantageous than fasting plasma C-peptide in connection with metabolic control measured as HbAlc and daily insulin dose in insulin-treated diabetic patients. Even though it can provide an integrated measure of B-cell secretory function throughout 24-h period19), 24-h urinary C-peptide may not be a better indicator in relation to metabolic control.