A Case of Massive Cisplatin Overdose Managed by Plasmapheresis
Article information
Abstract
Objectives:
Accidental cisplatin overdose occurs with increasing frequency despite the safeguards taken in prescription and administration, since cisplatin has been used increasingly for the treatment of numerous malignancies. Accidentally, a 59-year-old male received massive cisplatin overdose of 300mg/m2.
Methods:
Laboratory documentation included measurement of cisplatin concentrations by flameless atomic absorption spectroscopy(Varian, Spectra AA 300).
Results:
Toxicities included severe emesis, myelosuppression, renal failure, mental deterioration with hallucination, dim vision and hepatic toxicity. Plasmapheresis was effective in lowering the platinum concentration from greatest 2979 ng/ml to 185 ng/ml and appeared to be of clinical benefit.
Granulocyte-macrophage colony stimulating factor(GM-CSF) was used to ameliorate myelosuppression. The patient's renal function was restored 3 months later and partial response of esophageal cancer was obtained.
Conclusions:
Plasmapheresis was effective in lowering the platinum concentration in massive cisplatin overdose. This case heightens awareness to the possibility of accidental cisplatin overdose and the benefits of prompt management.
INTRODUCTION
Cis-diamminedichloroplatinum(II)(cisplatin) is administered at doses of 50–100mg/m2 in conjunction with intravenous hydration. Split dose is also administered to reduce the toxicities. This article report a 59-year-old man who received a massive cisplatin overdose 300 mg/m2 with hydration and managed by plasmapheresis and GM-CSF and who fully recovered from toxicities.
CASE REPORT
A 59 year-old-man with Stage III esophageal squamous cell carcinoma was treated with neoadjuvant 5-Fluorouracil(5-FU) and cisplatin 3 cycles followed by radiotherapy in another hospital. He achieved partial remission. Then disease progression was noted 3 months later.
Chemotherapy with 5-FU 1000mg/m2 D1–5 and cisplatin 100mg/m2 in 5 divided daily doses was reinstituted, but accidentally cisplatin was ordered to be administered daily for 5 days.
On day 3, he began to experience protracted nausea and vomiting, visual dimness and visual hallucination was noted with somnolence and general weakness. After reviewing the drug chart, we found cisplatin was given at 100mg/m2 daily for 3 days instead of given in 5 divided daily dose i. e. 20mg/m2 daily for 5 days.
We also noted diminished vision but ophthalmoscopic examination showed normal fundus and no papilledema. There was peripheral neuropathy.
On day 4, GM-CSF 250ug/m2(400ug) was started.
On day 6, Ccr was 21.4ml/min, aspartate transaminase/alanine transaminase(AST/ALT) were 1127/663 U/L, respectively. Plasmapheresis was initiated in an effort to remove platinum from the patient.
On day 15, leukocyte count was 1700/mm3 (granulocyte 63%), fever was sustained and third generation cephalosporin and aminoglycoside were started.
On day 16, septic thrombophlebitis of left forearm was noted and blood culture revealed the growth of Staphylococcus auricularis.
On day 22, agranulocytosis(WBC 600/mm3, with no granulocyte) was noted but renal failure and septic condition were restored slowly and swallowing difficulty was also subsided and he was able to swallow solid food without any difficulty, and esophagogram showed good passage of barium meal compared to that of before the chemotherapy. Renal failure was also recovered completely and hearing loss was, too.
METHODS
Measurement of Platinum Concentration
Platinum concentrations are measured by flameless Zeeman atomic absorption Spectrophotometry1) by Varian, Spectra AA 300, Sunkyung Industry, Suwon, KyungKiDo.
Samples were obtained from blood, urine and postpheresis plasma. Blood samples were collected in heparinized or acid citrate dextrose tubules, spun in clinical centrifuge within 10 minutes of collection, immediately separated as plasma samples and sent for analysis.
RESULTS
Removal of Cisplatin from the Patient
Cisplatin rapidly forms bifunctional cross-links with any nucleophilic molecule, so nucleophilic sulfur thiols can inactivate cisplatin and thus act as chemoprotectants2). But, intracellular thiol, glutathion cannot be available to bind with cisplatin which binds to target sites on proteins and DNA. Exogenously administered thiols could not be effective.
In a previous case3), dialysis was ineffective in lowering plasma platinum concentration because platinum binds to plasma protein very rapidly.
Chu et al proved the effectiveness of plasmapheresis in lowering the plasma platinum concentration4). In this case, the overdosing of cisplatin was found 7 days earlier than Chu's case found on day 10.
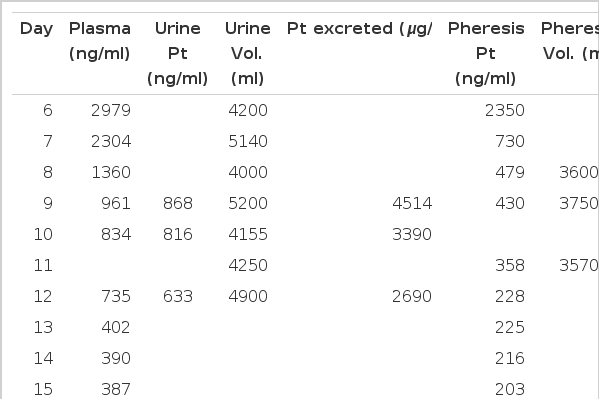
The patient was treated with plasmapheresis, which proved to be effective in lowering the plasma platinum concentration(Table 1 and Fig. 1).
From day 6 through 9, the patient received plasmapheresis 4 times and the plasma platinum concentration decreased from 2979ng/ml to 430ng/ml and he became very alert and did not have nausea and vomiting, but plasma platinum concentration increased to 834ng/ml on day 11 (Table 1). Although cisplatin rapidly became protein bound, this result suggests that there was a pool of exchangeable cisplatin that could move onto new plasma protein binding sites. Thus plasmapheresis was continued through day 16, a total of 10 times. Then the plasma platinum concentration gradually declined. Significant amounts of platinum were excreted in the urine in the setting of the patients non-oliguric renal failure. Thus urine output was increased by careful intravenous hydration(Fig. 4).
Figure 4 shows the effect of urine volume on platinum clearance and platinum clearance increased linearly with urine volume(r=0.0496 by linear regression).
DISCUSSION
Cisplatin toxicities were severe emesis, myelosuppression, nephrotoxicity and ototoxicity which are seen even in patients receiving usual doses. Others are central neurotoxicity, visual loss and hepatic toxicity which were unusual and probably caused by the magnitude of the overdose.
The severe myelosuppression was complicated by life-threatening sepsis. Thus GM-CSF was instituted 1 day after the stopping of cisplatin. Even though GM-CSF was administered at the earliest possible, nadir leukocyte count was 600/mm3 without granulocyte on day 22, and since then recovered fully on day 33.
The most serious and usually dose limiting toxicity of cisplatin is renal. This toxicity is manifested clinically by elevated BUN and creatinine, is cumulative with continued cisplatin exposure and is potentiated by other nephrotoxins5).
Non-oliguric acute renal failure was developed. The creatinine clearance (Ccr) was 21.4ml/min on day 6 and decreased to 8.3ml/min on day 13, but slowly recovered without hemodialysis, so Ccr was 36.6ml/min on day 34. High fluid intake with forced diuresis can reduce the incidence and severity of the renal toxicity6).
Systemic administration of thiols can reduce renal toxicity of cisplatin in animal models, and in clinical trial systemic diethyldithiodicarbamate appeared to reduce nephrotoxicity without affecting ototoxicity or myelosupression7). In this case, N-acetylcystein(Muteran®) and mesna were also administered with uncertain efficacy 2 days after cisplatin overdose.
Even though cisplatin induced deafness is seen at conventional doses, it is more pronounced in elderly patients8). This case had transient auditory dimness, but fully recovered.
Ototoxicity is characterized by tinnitus and hearing loss9). The hearing loss is usually in the high frequency range, 4000–8000Hz, but may occur in the lower ranges, which include the speech frequency10). Since the higher frequencies are usually involved, the hearing loss may not be symptomatic. Vestibular toxicity does not usually occur, but can be seen11).
The neurotoxicity, seen with the administration of cisplatin, consists principally of peripheral neuropathy involving both the upper and lower extremities with paresthesias, weakness, tremors and loss of taste12). Seizure and leukoencephalopathy have also been described13). Central nervous system toxicities were visual hallucination, somnolence without seizure on day 3, and peripheral neuropathy was also noted because of previously received cisplatin.
The neurotoxicity has become the dose limiting toxicity of cisplatin14). An interesting observation is that treatment of animals with an ACTH analogue will prevent neurotoxicity from cisplatin, and will facilitate the recovery of established neurotoxicity15), but does not interfere with the antitumor effect of the agent.
Loss of vision was reversible. After 1 month, visual acuity was recovered. Visual toxicity after high dose(200mg/m2) cisplatin therapy has been reported previously16).
Visual evoked potentials were normal, and loss of vision primarily was attributable to retinal damage, leading to blurred vision and altered color perception.
Abnormal elevation of transaminase and prothrombin time and partial thromboplastin time was noted. The peak abnormalities were on day 6 and resolved by day 9. The abnormalities in clotting times may have been the result of liver dysfunction and concomitant impaired synthesis of clotting proteins, but also could have been a direct effect of cisplatin binding to the clotting proteins.
Although protein bound platinum is less reactive than the free form, there appeared to be an exchangeable pool of platinum. Chu et al4) observed that clotted blood has low plasma platinum concentration and gradually increased when plasmapheresis was not given, and continued cellular response to cisplatin was measured by a markedly decreased level of XPE binding factor in extractable peripheral blood lymphocytes and supported the presence of an exchangeable pool of platinum.
After most of the cisplatin has become protein bound, high plasma platinum concentration might be capable of exerting biological effects.
As Chu et al suggested, enhancing the clearance of platinum by removing platinum from an exchangeable pool, that was capable of producing additional toxicity, was beneficial to improve the toxicities.
In this case, early trial of plasmapheresis was very effective and shortened the period of non-oliguric renal failure without dialysis. Cisplatin poisoning with 200 mg/m2 and 480mg/m2 with hydration managed by hemodialysis for renal failure were reported3,17). But hemodialysis was ineffective in lowering the plasma platinum concentration.
Chu et al first treated cisplatin 280 mg/m2 overdose and also reported that the use of the growth factor GM-CSF appeared to be safe in attempting to shorten the period of myelosuppression, despite the continued presence of a significant level of cisplatin4).
We report the first case in Korea for cisplatin poisoning successfully managed by plasmapheresis in lowering the plasma platinum concentration.
The Bristol-Myers Squibb spontaneous Adverse Drug Reaction Reporting System reported the three types of dosing errors to have caused the cisplatin overdoses18).
(1) Mistaking dosing orders when they are written as the total dosage divided over a period of several days; (2) administering cisplatin when the patient was to have received carboplatin; (3) writing the wrong cisplatin dose.
It recommended that to minimize the possibility of error, the following procedures should be considered19): (1) Always write for the intended daily dose of cisplatin rather than the total dose over a period of time; (2) the institution of an alerting system for orders of cisplatin exceeding 120mg/m2 per course.
This case illustrates the need for careful monitoring during administration of any cytotoxic agents and shows that errors can occur, even in a center experienced in the use of high-dose cisplatin. It also suggests that early trial of plasmapheresis can ameliorate the fatal toxicities of cisplatin overdose.
Acknowledgements
We would like to thank Mr. Cho, Yong Back of Sunkyung Industry Research Center for his assistance in measuring plasma platinum concentration.