The Changes of Serum-ACE, Plasma Renin Activity and Aldosterone in the Diabetics with Hypertension
Article information
Abstract
It is well known that the serum-ACE levels are elevated in sarcoidosis, Gaucher’s disease, and some granulomatous diseases. Recently, the serum-ACE level was also found to be elevated in diabetes mellitus. To confirm the possible role of plasma renin activity and aldosterone in the pathogenesis of hypertension and to assess the relationship of serum ACE to the plasma renin activity and aldosterone profiles in diabetics, we measured the levels of serum ACE, plasma renin activity, aldosterone and blood pressure in 38 diabetic patients who were admitted to Kyung Hee University Hospital from June 1981 to September, 1982. The results were as follows:
The S-ACE activity was significantly elevated in 38 patients with diabetes mellitus (9.42±6.60 Unit) as compared with that in the healthy controls (2.53±1.54 Unit) (p<0.005) (p<0.005).
The S-ACE activity was significantly elevated in 15 hypertensive diabetics (14.29± 7.10 Unit) as compared with that in 23 normotensive diabetics (6.24±3.68 Unit) (p<0.005).
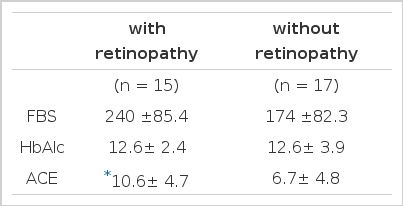
The S-ACE activity was elevated in 15 diabetic patients with retinopathy (10.6±4.7 Unit) as compared with the levels in 17 diabetic patients without retinopathy (6.7±4.8 U) (p<0.05).
The S-ACE activity was directly correlated to the mean arterial pressure (r=0.54) (p<0.005).
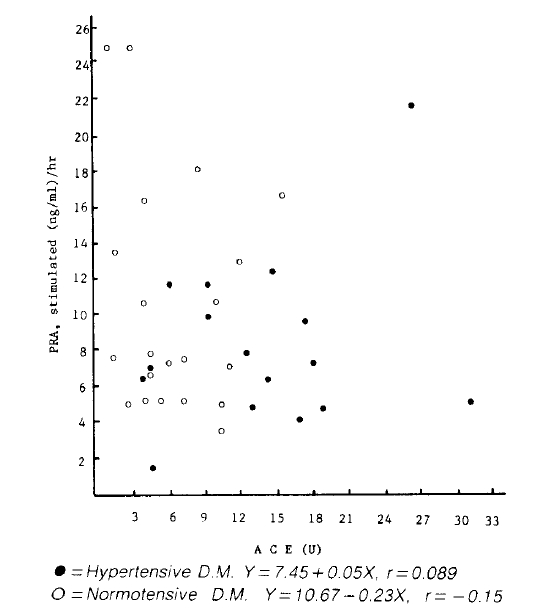
The plasma renin activity and aldosternone concentrations in diabetics were not different from the normal controls and had no correlationship to the S-ACE activity.
After 2 weeks of insulin therapy in 15 diabetics, the S-ACE activity was not changed.
INTRODUCTION
In 1974 Lieberman reported that serum angiotensin converting enzyme (S-ACE) activity increased in sarcoidosis.1) Thereafter, increased S-ACE activity has been reported in Gaucher’s disease,2,3) hyperthyroidism,4) other granulomatous diseases5) and also in diabetes mellitus.6) We previously reported that S-ACE activity significantly increased in diabetics with retinopathy compared to those without retinopathy.7)
Because many diabetic patients have hypertension, we observed the change of S-ACE activity in hypertensive diabetics in order to test the hypothesis that increased S-ACE activity may play a role in the pathogenesis of hypertension in diabetics.
SUBJECTS
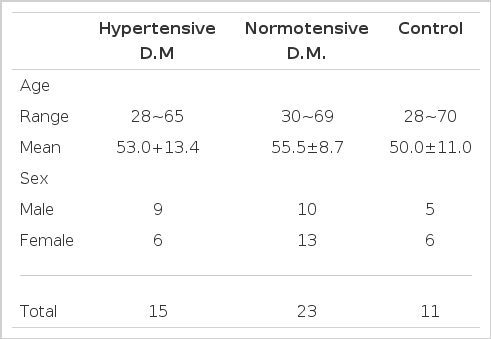
Serum or plasma was collected from 38 diabetics and 11 healthy normal controls. Diabetic patients included 38 non-insulin dependent diabetes with normal renal function, aged from 28 years to 65 years, and were classified into two groups, normotensive and hypertensive (> 150/90 mmHg), by blood pressure. The mean ages were not different between the two groups and normal control group (Table 1).
METHOD
S-ACE activity was measured by the modified method of Lieberman et al.8) Plasma renin activity (PRA) and aldosterone concentration were measured by radioimmunoassay. Stimulation of PRA and aldosterone was done by furosemide injection (40 mg bolus IV) after withdrawal of venous blood of basal status on supine position at 6 a.m.
RESULTS
Mean S-ACE level of 38 diabetics was 9.42 ± 6.60 Unit and significantly higher than that of the healthy controls (2.53±1.54 Unit).
The mean S-ACE activity significantly increased (14.29 ±7.10 Unit) in 15 hypertensive diabetics as compared to that of 23 normotensive diabetics (6.24 ±3.68 Unit) (p<0.005).
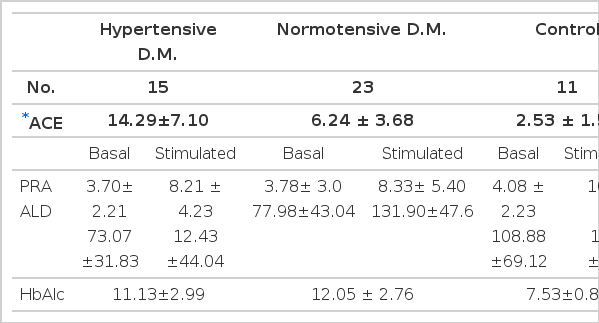
Both basal and stimulated PRA and aldosterone concentrations were not different between two diabetic groups and also not different from those of normal controls (Table 2).
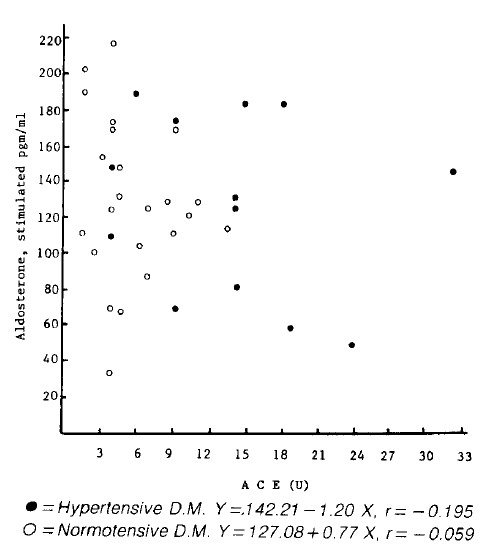
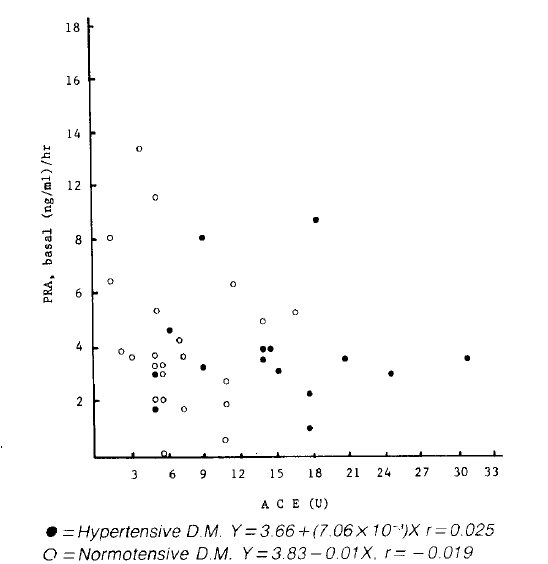
There was no significant correlation between S-ACE activity and both basal and stimulated plasma aldosterone concentration in both hypertensive diabetics and normal controls (Fig. 4, 5). Also, there was no significant correlation between S-ACE activity and PRA in both diabetics and normal controls (Fig. 2, 3).
Mean arterial pressure correlated well with S-ACE activity in 38 diabetics (r = 0.54 p<0.005) (Fig. 1).
In 15 diabetics who were diagnosed as having retinopathy by ophthalmologists, S-ACE activity was significantly higher (10.6 ±4.7 Unit) than that of diabetics without retinopathy (6.7 ± 4.8 Unit) (p<0.05) (Table 3).
S-ACE activity after two weeks of insulin therapy did not change in 15 diabetic patients with retinopathy (7.42 ±2.72 Unit vs 7.29 ± 4.14 Unit) as compared to that before therapy (Table 4).
DISCUSSION
Angiotensin converting enzyme is a zinc containing dipeptidyl carboxypeptidase with a molecular weight of approximately 129,000. It consists of 837 aminoacids with threonine on its aminoterminal and alanine on its carboxy terminal.9,10) ACE catalyzes the release of histidylleucine from the carboxy terminal of angiotension I to yield a pressor octapeptide, angiotension II, and on the other hand it inactivates bradykinin, vasopressor nonapeptide, by catalyzing the cleavage of its carboxy terminal.11–15) This enzyme exists mainly in the vascular endothelial cells of the lung and the distal tubular epithelial cells of the kidney.16)
In 1979 Hinman et al. reported that this enzyme was also found in the human alveolar macrophage.17–18) Particularly S-ACE activity in the lung tissue is 30 times as high as that in the blood.19,23) The elevation of S-ACE activity in sarcoidosis may be due to release from the epithelial cells of sarcoid granulomata.20) The elevation of S-ACE activity in patients with hyperthyroidism has been thought to be due to excessive hormones directly or due to increased release from the endothelial cells indirectly.4) Therefore, it seems that S-ACE activity can be increased in all diseases which have the proliferation of endothelial cells and S-ACE is closely related to the change of the endothelial cells.
In 1980 Lieberman et al. reported that S-ACE activity increased in the diabetic patients with retinopathy and suggested that S-ACE activity was correlated with and might play a role in diabetic vascular complications.6)
It has been well known that diabetes mellitus is usually accompanied by hypertension. The prevalence of hypertension among the diabetics has been reported in the range of 41 % to 80%. But the mechanism for the development of hypertension in diabetics remains unclear. Various factors, mainly diabetic glomerulosclerosis and other vascular changes due to arteriosclerosis, may account for increased prevalence of hypertension in diabetics.21)
Although the role of renin-angiotensin system, adrenocortical hormone and prostaglandin, the definite mechanism remains unclear. Most of diabetics with hypertension have an increased concentration of plasma aldosterone and a decreased PRA.22) So we investigated the changes of PRA and aldosterone and their relationship to the change of S-ACE activity.
The S-ACE activity was significantly elevated in hypertensive diabetics as compared with the level in normotensive diabetics, but PRA and plasma aldosterone concentration did not change in both group and were not correlated to the S-ACE activity. It is not clear whether the elevated S-ACE level is the primary cause for the development of hypertension in diabetics or the result of vascular injury caused by diabetic complications. But the higher prevalence of hypertension in older patients implies that the vascular change precedes hypertension. Lieberman et al. demonstrated that subjects with low S-ACE activity did not have lower pressure than those with high S-ACE activity and S-ACE of the lung is normally present sufficiently and he suggested that the limiting factor regulating blood pressure would be the renin activity and the production of angiotension I.1) And they also asserted that hypotension would occur if S-ACE activity were totally absent but normal range of the enzyme activity should not have any effect on the systemic blood pressure.1) The report suggested that the elevated S-ACE activity is not a direct cause of the development of hypertension in diabetics. On the contrary, Campbell suggested that S-ACE activity was significantly higher in rabbits with many arterial lesions than that of the rabbits with few arterial lesions after inducing renal hypertension and therefore S-ACE might play a pathogenic role in the development of vascular lesion.1) But we suggested that S-ACE activity has no effect on the renin-angiotension system in the development of hypertension in diabetics because there was no correlation between the S-ACE activity and PRA or aldosterone concentration. In our results, we agree with the results of Lieberman that the increased S-ACE activity is not a direct cause of the development of hypertension in diabetics. It seems that the increased activity of S-ACE is the result of the release from the damaged vascular endothelium caused by hypertension.