Myelodysplastic Syndrome with Erythroid Aplasia following Pure Red Cell Aplasia
Article information
Abstract
Myelodysplastic syndrome (MDS) with erythroid aplasia is a very rare disorder that has not been clearly defined. We experienced a case of pure red cell aplasia (PRCA), which evolved to MDS with erythroid aplasia. A 59-year-old male with transfusion-dependent PRCA was referred to our hospital for an evaluation of newly developed thrombocytopenia. Two years ago, PRCA was diagnosed by the laboratory findings and a bone marrow examination, which showed no evidence of any myelodysplastic features and thymoma. Upon admission, the bone marrow findings showed marked hypercellularity, with numerous dysplastic features in the three lineages including erythroid hypoplasia. These findings were compatible with a diagnosis of MDS with red cell aplasia. It is very interesting that the PRCA evolved to MDS with red cell aplasia, which strongly suggests an autoimmune mechanism for the development of MDS.
INTRODUCTION
Pure red cell aplasia is a relatively uncommon disorder that is characterized by normochromic normocytic anemia, reticulocytopenia, and the absence of mature erythroid precursors in an otherwise normocellular bone marrow1, 2). The usual etiologies of PRCA have been reported an immunological or viral attack to the erythroid precursors. In rare circumstances, PRCA can be the only or initial manifestation of myelodysplasia, which rarely responds to immunosuppressive agents3, 4). Therefore, the most frequent and important hematological disorders from which PRCA differentiates to is myelodysplastic syndrome (MDS).
The median survival of patients with primary acquired PRCA has been reported to be approximately 10 years. The evolution of PRCA to aplastic anemia and acute nonlymphoblastic leukemia has been described and is regarded to be a serious complication5, 6).
However, reports on the evolution of PRCA to MDS with erythroid aplasia are rare. We experienced an interesting case with pure red cell aplasia (PRCA) that evolved to MDS with erythroid aplasia, and report this case with review of the relevant literature.
CASE REPORT
A 59-year-old male with a two years history of transfusion-dependent anemia was referred to our hospital for an evaluation of newly developed thrombocytopenia. Two years prior, the patient visited another hospital complaining of progressive weakness PRCA was diagnosed with a hemoglobin level of 3.8 g/dL, a white cell count of 2.29 × 109/L, and a platelet counts 301 × 109/L. and a reticulocyte count of 0.6%. The anti-nuclear antibody test was positive with a diffuse pattern and the anti-DNA antibody test as well as the anti-platelet antibody test was also positive. The bone marrow was normocellular with an increased M:E ratio of 12:1, and there was no evidence of myelodysplastic features or thymoma was not observed (Figure 1). Upon admission to our hospital, the peripheral blood cell count was as follows: Hb 3.7 g/dL, MCV 86.9 fL, MCH 31.9 pg, MCHC 36.7%, WBC 5.4 × 109/L with blasts of 2%, and a platelet count ofs 10 × 109/L. The bone marrow was markedly hypercellular with blasts of 3.2% and normoblasts of 0.2. The M:E ratio was 232.5:1. Numerous dysplastic features were observed in three lineages (Figure 2). The conventional cytogenetic analysis of the bone marrow cells showed a normal karyotype (46XY). The anti-nuclear antibody test was 1:1280 positive with a diffuse pattern in the interphase cells. The anti-platelet antibody test was also positive. The erythropoietin level was higher (168.44 mLU/mL). The direct and indirect antiglobulin tests were both negative. The HBs antigen test was negative and the antibody was positive, the HCV antibody test was negative. The Pavovirus B19 immunoglobulin G and M tests were both negative. These findings are compatible with a diagnosis of MDS with red cell aplasia. The patient was treated with high dose immunoglobulin and prednisolone. However, the thrombocytpenia did not improve with these treatments. Two months later, he refused further treatment and was discharged.
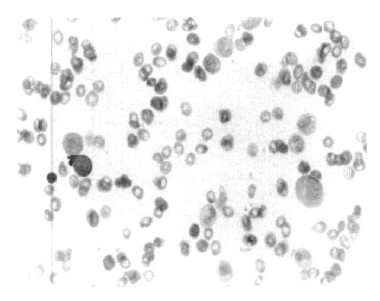
Bone marrow smear finding at the time of the pure red cell aplasia shows erythroid aplasia diagnosis, Wright-Giemza, ×400.
DISCUSSION
Although PRCA is a well-defined clinical disorder, its clinical manifestation is heterogeneous and its underlying pathogenesis varies. But, there have been a wide variety of immunological disturbances including antibodies to erythroblasts, erythropoietin-responsive cells, erythropoietin, and T cell suppression of the erythropoiesis7). The demonstration of such immune pathogeneses provides a rationale for its treatment with immunosuppression. Moreover, clinical remissions with immunomodulatory strategies have been reported.
In MDS, the hematopoietic precursors are dysplastic and proliferative with defective maturation. A variant of myelodysplasia, in which the neoplastic progenitor cell has a limited differentiation capacity that leads to a clinical phenotype that is indistinguishable from aplastic anemia or its variants, PRCA, has been described8). Usually these patients are unresponsive to immunosuppressive therapies and progress to nonlymphocytic leukemia5).
Myelodysplastic syndrome (MDS) with erythroid aplasia is a very rare disorder that has not been well defined but has been reported be one of the poor prognostic factors in MDS3). There are several case reports of myelodysplastic syndrome with erythroid aplasia, in whichthe marrow findings of MDS may be indistinguishable from that of typical PRCA3, 4).
In our case, there was no evidence of MDS, such as dysgranulopoiesis or dyserythropoiesis, on the initial examination of bone marrow two years ago when PRCA was diagnosed at other hospital. It is very interesting that the PRCA evolved to MDS over the subsequent two years, which suggests an autoimmune mechanism in the development of MDS with erythroid aplasia. A review of literature did not identify another such case, and the pathogenesis remains to be defined. Some investigators reported that an in vitro bone marrow culture from MDS patients, which is used to determine if the burst forming unit-erythroid (BFU-E) develops, is a useful method to distinguish between MDS and PRCA3). We did not perform such a study in our case. However, there was no evidence of MDS at the initial examination and some autoimmune features such as positive anti-nuclear antibody and anti-platelet antibody test, which were persistently positive at the time of diagnosis. This strongly suggest an autoimmune mechanism for the evolution from PRCA to MDS with erythroid aplasia rather than a clonal disorder or an intrinsic defect of the stem cells9, 10). If so, this might contribute to the development of a treatment strategy for MDS with erythroid aplasia.
