Detection of Epstein-Barr Virus by PCR and Expression of LMP1, p53, CD44 in Gastric Cancer
Article information
Abstract
Background :
Epstein-Barr virus (EBV) is associated with various lymphoproliferative disorders and nasopharyngeal carcinoma. Recently, some gastric cancer cells were observed to contain the EBV sequence. We detected EBV in gastric cancer by using PCR to determine the frequency of EBV-associated gastric cancer, and performed immunohistochemical staining for the latent membrane protein (LMP1), p53 and CD44 to investigate the possible mechanism in EBV-associated gastric cancer.
Methods :
Eighty-seven formalin-fixed and paraffin-embedded blocks (40 gastric adenocarcinomas, 34 adjacent normal tissues, 13 metastatic lymph nodes) from 40 surgically resected gastric specimens were studied. All patients were diagnosed with gastric cancer at the Kang-Nam St. Mary’s Hospital between April 1995 and April 1997. After DNA was extracted from each paraffin block, we performed PCR and immunohistochemical staining for the LMP1, p53 and CD44.
Results :
EBV was detected in 4 of 40 cases (10%). In 1 of 4 EBV-positive cases, EBV was also detected in a metastatic lymph node. The immunohistochemical staining for the LMP1, p53 and CD44 were negative in all the EBV-positive cancer patients. Of the patients having these cancers, 2 had a poorly differentiated adenocarcinoma with a lymphoepithelioma-like morphology.
Discussion :
The frequency of EBV-associated gastric cancer is about 10% in Korea. Considering the negative result of the immunohistochemical staining for the LMP1, p53 and CD44, EBV-associated gastric cancer seems to have a different mechanism of tumorigenesis from ordinary gastric cancer or other EBV-associated cancers. This specific mechanism must be determined by further large scale studies.
INTRODUCTION
Epstein-Barr virus (EBV) is associated with a variety of lymphoproliferative disorders, such as Burkitt’s lymphoma, Hodgkin’s disease, T-cell lymphoma, B-cell lymphoma in immunodeficiency, and lymphoepithelioma-like carcinoma occurring in the parotid gland, thyroid, lung, and nasopharynx1,2).
Since Burke et al first detected EBV in undifferentiated lymphoepithelioma-like gastric cancer by polymerase chain reaction (PCR) in 19903), many authors reported the association between EBV and gastric cancer with lymphoepithelioma-like lesions. EBV-associated gastric cancer is characterized by prominent lymphocyte infiltration around the tumor, resembling typical nasopharyngeal lymphoepithelioma. If this typical lymphoepithelioma-like lesion is present in gastric cancer, the EBV detection rate may be as high as from 77.8% to 100%4,5). It was recently reported that EBV may be detected in ordinary gastric cancer without the presence of lymphoepithelioma-like lesions, but its incidence is lower than that of gastric cancer with lymphoepithelioma-like lesion, from 1.8% to 16%5,6).
Although there have been many reports on the EBV association with gastric cancer, it is not clear how EBV plays a role in cancer development.
Gastric cancer is the most common cancer, and is the leading cause of cancer-related death in Korea. We detected the EBV in gastric cancer by using PCR to determine the frequency of EBV-associated gastric cancer in Korea and performed immunohistochemical staining for the latent membrane protein (LMP1), p53, and CD44, which are known to be involved in cancer development, in order to investigate the possible role of EBV in gastric cancer.
MATERIALS AND METHODS
1. Specimens
Formalin-fixed and paraffin-embedded blocks from eighty-seven surgically resected gastric specimens (40 cases with gastric adenocarcinoma, 34 normal tissue adjacent to tumor, 13 metastatic lymph nodes) were studied. Clinical data were obtained from clinical records.
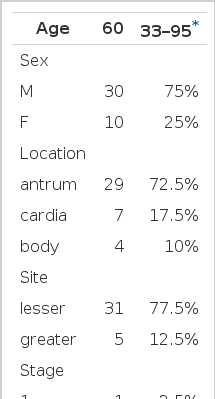
A total of 40 cases (30 men and 10 women) were studied. All patients were diagnosed with gastric cancer between April 1995 and April 1997 at Kangnam St. Mary’s hospital. Median age was 60 years. 29 cases (72.5%) were in the antrum and 7 cases (17.5%) in the cardia. Most lesions were located at the lesser curvature (77.5%) and 5 cases in the greater curvature (Table 1). According to the Lauren classification7), 40 cases consisted of 20 intestinal type, 10 diffuse type, and 10 mixed type. 22 patients had moderate differentiated adenocarcinoma, and 14 patients had poorly differentiated adenocarcinoma. Lymphatic invasion was found in 34 cases (85%), and vascular invasion was found in 8 cases (20%) (Table 2).
We obtained sixteen benign ulcer tissues from gastrofiberscopic biopsy specimen at St. Mary’s hospital between February 2000 and March 2000 as the control group, and subjected to these specimens to the same procedure.
2. PCR
Paraffin-embedded blocks were cut into 10 μm thick sections and H-E stained for microdissection of the tumor tissue under light microscopy. These microdissected tumor tissues were put into 1.5 mL microcentrifuge tubes, and were treated with 0.5 mL TaKaRa DEXPAT™ (cat. #9091, TaKaRa, Japan) to extract DNA as the manufacturers’ instructions. After incubation at 100°C for 10 minutes, the samples were centrifuged at 12,000 rpm for 10 minutes. The supernatant containing DNA was stored at −20°C and then used as the template for the PCR. The primer for PCR was a 129 bp region of the internal repeat, EBNA-1 (Epstein-Barr virus nuclear antigen-1), described in Table 3. The template DNA 1 μg was mixed with 10 X buffer (100 mM Tris-HCl, 500 mM KCl, 15 mM MgCl2), 10 mM dNTP mixture, and 1.5 μU Taq polymerase (TaKaRa, Japan). We added the primer to this mixture, and then performed PCR (MJ Research Inc. USA). The prepared samples were denatured at 95°C for 5 minutes and were subjected to 35 cycles of amplification. Each cycle consisted of 30 seconds of denaturation at 95°C, 30 seconds of annealing at 58°C, and 1 minute of extension at 72°C. Finally, the mixture samples were extended at 72°C for 5 minutes. We used the B95-8 cell line (EBV-transformed leukocytes) as a positive control, and a mixture of samples without DNA served as a negative control. The products of PCR were electrophoresed in a 2% agarose gel, and then stained with ethidium bromide.
3. Immunohistochemical staining
We used CS1-4 monoclonal antibodies (DAKO, clone CS1-4, Denmark) for the LMP1, DO-7 monoclonal antibodies (DAKO, clone DO-7, Japan) for p53, and the variant-specific MAbs (R&D system, Abingdon, UK) for CD44. Formalin-fixed, paraffin-embedded sections were dewaxed and rehydrated in graded alcohol. After ending endogenous peroxidase activity with H2O2, sections were microwave-irradiated for 10 minutes in pH 6.0 citric acid buffer. After incubating for 120 minutes at room temperature, sections were mixed with pepsin for 10 min, and then added to acid alcohol. A primary antibody was applied for 30 minutes at 40°C and then rinsed with automaffin buffer. After the secondary antibody was applied for 10 minutes at 40°C, the avidin-alkaline phosphate procedure was applied for 15 minutes at 45°C. Finally, the sections were visualized by chromogen at 45°C and counterstained by Hematoxylin. The antibody for LMP1 was diluted 1:100 and other antibodies were diluted 1:50. The result was interpreted by two pathologists separately.
RESULTS
1. EBV PCR & Immunohistochemical staining
EBV was detected in 4 of 40 cases (10%) of gastric adenocarcinoma by PCR (Figure 1), but not in the benign ulcer cases.
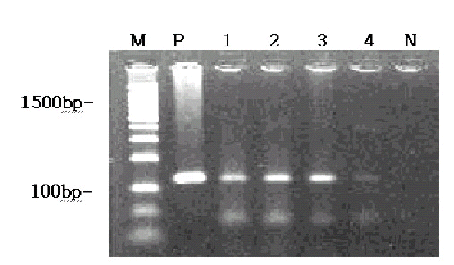
Detection of EBV DNA by PCR, corresponding to the IR region.
M: Marker (100bp DNA ladder), P: positive control (B95-8 cell line; 129bp), 1, 2, 3, 4: tumor tissue, N: negative control (blank).
With regard to LMP1, CD44 and p53 expression, all 4 EBV-positive cases were negative, but 9 and 15 cases were positive for p53 and CD44, respectively, in the 36 EBV-negative group (Table 4). However, EBV was not detected in 34 adjacent normal tissues and all these cases were negative in immunohistochemical staining. EBV was detected in only 1 of 13 metastatic lymph nodes, and that was from an EBV-positive case (Table 5).
2. Clinical characteristics of EBV-positive cancer
In the 4 EBV-positive cases, 3 patients were men and other 1 patient was a woman. The lesions were located at the lesser curvature (2 antrum and 2 cardia). Histologically, 2 cases were moderately differentiated carcinoma and the other 2 cases were poorly differentiated carcinoma with lymphoepithelioma-like lesions (Table 6).
DISCUSSION
In this study, we extracted DNA from gastric cancer specimens and detected EBV by PCR in order to determine the frequency of EBV-associated gastric cancer in Korea. We also performed immunohistochemical staining in an attempt to investigate the role of EBV in cancer development. We used PCR to detect EBV because low copies of EBV DNA are hard to detect with in situ hybridization, but can be detected by PCR8). The frequency of EBV-associated gastric cancer was about 10% in our study, which was similar to results reported in Hong Kong, China and Japan. No EBV was detected in adjacent normal tissue among the 4 EBV-positive group, but EBV was found in 1 metastatic lymph node in the EBV positive group. Considering this result, we hypothesized that EBV may have an association with gastric cancer, and is not accidentally found in gastric cancer.
LMP1, an integrated protein expressed in the cell membrane of EBV, has been known to play a major role in cancer development. If LMP1 is transfected into rodents, it prevents cells from apoptosis to produce immortalized cells9–10). Shin et al reported that LMP1 was expressed in 3 of 12 EBV-positive gastric cancers in their study11), and Ham et al reported LMP1 expression in 2 of 6 cases12). However, LMP1 expression has rarely been described in EBV-associated gastric cancer13). Our study also showed that all 4 EBV-positive cases were negative in LMP1 expression. Some authors suggested that the low expression of LMP1 in EBV-associated gastric cancer may be due to the technical limitations of the methods used11, 13). However, Sheu et al suggested the following hypothesis: LMP1 expression can be repressed in EBV-associated gastric cancer, and it can make tumor cells evade immune surveillance by the host immune system. Then, tumor cells can grow continuously to develop cancer without LMP1 expression14). Further investigation may be needed to specifically define the role of LMP1 in tumorigenesis in EBV-associated gastric cancer.
The p53 mutation may be frequently seen in various type of cancers, including lung, colon, as well as gastric cancer. Kim et al reported that the rate of p53 overexpression was 100% in EBV-positive gastric cancer, but 85% in EBV-negative gastric cancer15). In another report, p53 overexpression was also 58.8% and 47.7% in EBV positive and negative gastric cancer, respectively16). Therefore, there seems to be no significant difference in the p53 overexpression between EBV positive and negative gastric cancer. In our study, we found that p53 over-expression was negative in all EBV-positive cases, but positive in some EBV-negative cancer. So, we hypothesized that no direct relation exists between p53 mutation and EBV-associated gastric cancer, and there may be another mechanism for cancer development in EBV-associated gastric cancer, which is different from the cancer development in ordinary gastric cancer.
CD 44 is a cell surface glycoprotein that acts as an adhesion molecule. CD44 variant is associated with EBV infection and it shows correlation with lymph node metastasis17). In our study, CD 44 was not expressed in EBV-positive cancers but highly expressed in EBV-negative cancers. We require greater numbers of cases to further investigate and define the relation between EBV infection and CD44.
In 2 of 4 EBV-positive gastric cancer, histological findings were similar to ordinary gastric cancer, but the other 2 cases had distinct “lymphoepithelioma-like” lesions, that is prominent infiltration of lymphocytes around the tumor with poor differentiation. Because this lymphoepithelioma-like lesion was also found in 2 cases of EBV-negative cancer, it is not certain whether this lesion is a typical finding of EBV-associated cancer or a reactive lesion of lymphoid tissue to EBV infection18). The clinical characteristics of EBV-positive gastric cancer were not different from those of ordinary gastric cancer, although it is not entirely clear due to the small number of cases in our study. Since EBV-associated gastric cancers were known to be more prevalent in the upper and middle portions of the stomach than in the lower part, 2 of 4 cases in our study were located at the cardiac portion. Osato et al suggested the following hypothesis for this tendency: Orally excreted EBV, EBV-infected lymphocytes or oral epithelium is ingested. These particles stimulated gastric mucosa, and then LMP1 or another oncogene in the gastric mucosa becomes overexpressed to develop into cancer. But EBV may lose infectivity easily in the stomach, so the EBV-associated gastric cancer is more common in the upper part of the stomach19).
In summary, the frequency of EBV-associated gastric cancer in Korea is about 10%, less common than ordinary gastric cancer. If the lesion is located at the upper part and an lymphoepithelioma-like lesion is found in gastric cancer, we may suspect EBV-associated gastric cancer. Further study may be required to determine the clinical characteristics of EBV-associated gastric cancer, and we also have to investigate another possible mechanism of tumorigenesis to define the role of EBV in EBV-associated gastric cancer.