Severe Airway Hyperresponsiveness in School-aged Boys with a High Body Mass Index
Article information
Abstract
Background
An association between obesity and asthma has been reported. The prevalence of airway hyperresponsiveness (AHR), results of skin prick tests, body mass index (BMI), and asthma symptoms were examined in schoolchildren.
Methods
The results of BMI (kg/m2) determination, skin prick testing, spirometry, asthma questionnaires, and methacholine challenge tests were obtained in a cross-sectional survey of 667 schoolchildren. The methacholine concentration causing a 20% fall in FEV1 (PC20) was used as the threshold of AHR. If the PC20 was less than 16 mg/mL, the subject was considered to have methachloine mediated AHR.
Results
The mean BMI was 17.1±0.09 kg/m2. The prevalence of AHR was 42.7%. The sensitization rate to common inhalant allergens was 30.3%. PC20 in children with BMIs ≥17.1 kg/m2 was significantly lower than that in children with BMIs 17.1 kg/m2. The mean BMIs of boys and girls were not significantly different. The levels of PC20 by sex were not different. The children were grouped by sex into percentile of BMI. PC20 in boys was lower in the obese group than in the non-weight and overweight groups (p<0.05). PC20 in boys and girls with atopy was significantly lower than in those without atopy. In a multiple logistic regression model that included all of the children and adjusted for confounding variables, independent associations with AHR were seen with BMI, asthma symptoms, and atopy.
Conclusions
BMI had an association with AHR in school-age boys.
INTRODUCTION
The prevalence rates of asthma and obesity continue to rise. A number of environmental factors, including air pollution, cigarette smoking, allergen exposure, and diet have been proposed as explanations for the increases in the prevalence of asthma1). The prevalence of asthma2) has increased in Korea (from 5.7% in 1980 to 10.1% in 1990). Asthma and allergy in developing countries may be associated with the adoption of an urbanized "western" life style. The total intake of calories is increasing with the industrialization of Korea. Recent studies have found an association between body mass index (BMI) and asthma in young adults. A study of diet and asthma from Norway observed a positive relationship between BMI and asthma symptoms3). In a nationally representative British birth cohort, BMI was positively associated with the prevalence of asthma and wheezing in individuals studied at 26 years of age4). General obesity and central obesity are potential risk factors of asthma in relatively non-obese Korean adults5). BMI is associated with wheezing in older adults living in rural areas in Korea6). In the large Nurses' Health Study II in the USA, a strong positive association between BMI and the risk of incidence of asthma was observed in women aged 27-44 years who were followed over four years7). The effects of diet may be mediated through an increase in the synthesis of prostaglandin E2, which in turn can promote the formation of immunoglobulin E1).
Recent cross-sectional studies have suggested that there is an association between being overweight or obese and experiencing asthma symptoms. This association seems to be much stronger in females than in males, suggesting the possibility that female hormones may be directly involved in the putative causal pathway that relates obesity to asthma4, 8-11).
Therefore, we have examined the relationship between BMI and AHR, atopy, and asthma symptoms in schoolchildren.
MATERIALS AND METHODS
Study population and questionnaire
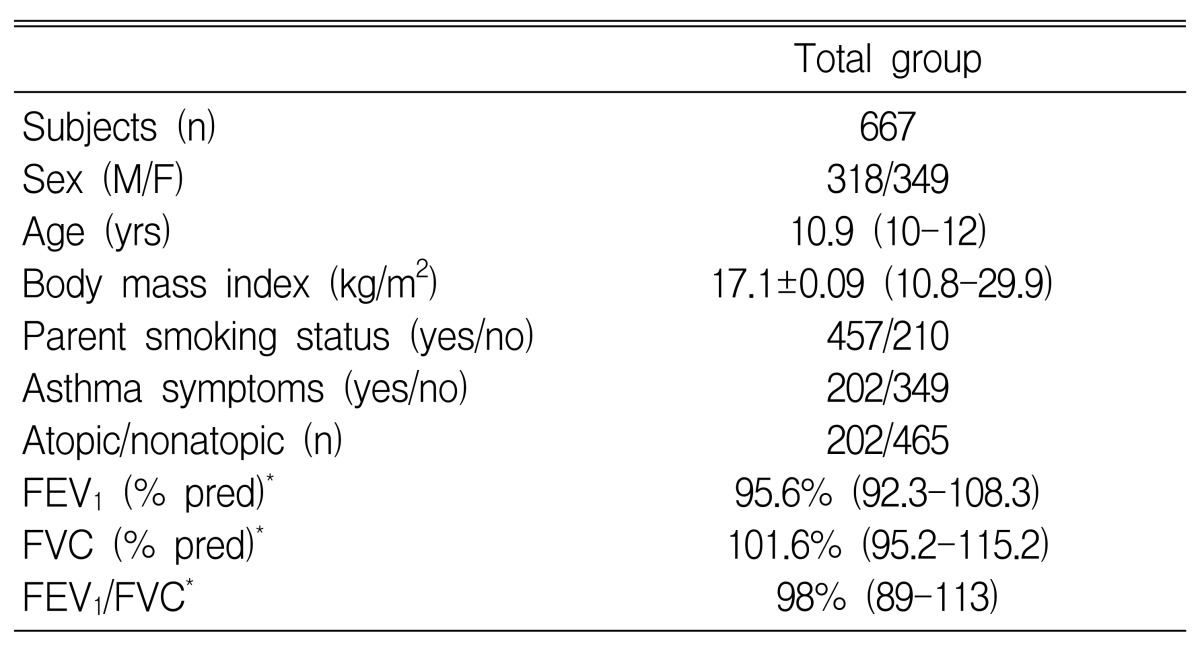
All children were 10 to 12 years of age living in both polluted and non-polluted areas. A questionnaire on respiratory and allergic disorders was administered that included questions developed for the International Study of Asthma and Allergies in Childhood (ISAAC). The focus of this survey was on responses to the ISAAC questions on "wheezing in the last 12 months", "number of wheezing attacks in the last 12 months", and on "sleep-disturbing" and "speech limiting" wheezing in the last 12 months. Symptoms of allergic rhinoconjunctivitis (sneezing or runny nose, or blocked nose without a cold and itchy-watery eyes) were assessed12). In addition, a question on morning coughing as a symptom of nonspecific airway irritation was asked ("Did you frequently cough in the morning right after waking up in the last 12 months?"). A total of 667 schoolchildren were enrolled in this study (Table 1). None of the subjects took any drugs such as anti-histamines, cromolyn, theophylline, or sympathomimetics that could interfere with the performance of skin tests or methacholine challenge test within 72 hours of the tests. This study was approved by the Research Committee of the Soonchunhyang University, and all guardians signed informed consent forms before the study.
Pulmonary function studies
Spirometry was performed with a SensorMedics 2200 spirometer (Cardiopulmonary Care Company™, Yorba Linda, California). Baseline measurements of VC and FEV1 were selected according to American Thoracic Society criteria11), and reference values were taken from Choi et al14).
Allergy skin testing
Allergy skin prick tests were performed with eleven common allergen extracts (Dermatophagoides farinae, Dermatophagoides pteronyssinus, Aspergillus spp, alder, birch, hazel, rye, timothy, mugwort, ragweed, Blatella germinica, histamine (1 mg/mL), and saline; Allergopharma, Germany). The reactions were read 15 minutes later. A wheal and erythema size equal to or greater than that of histamine (positive control) was read as 3+. Reactors were defined as exhibiting atopy when they showed a response >3+ to one or more allergens in the skin prick tests15).
Body mass index
The BMI for an individual was defined as weight (kg) divided by the square of height (m). The children were grouped into percentile of BMI. Children <85th percentile were "non-weighted", ≥85th to 95th percentile were "overweighted", and ≥95th percentile were "obese".
Airway hyperresponsiveness
Methacholine challenge tests were carried out by a modified method as described by Chai et al16). Concentrations of 0.075, 1.25, 2.5, 5, 10, and 25 mg/mL methacholine were prepared by dilution in buffered saline. A Micro-dosimeter (S&M Instrument Co., Doylestown, PA) was used to deliver the aerosol generated by a DeVilbiss 646 nebulizer. Subjects inhaled 5 breaths of increasing concentrations of methacholine until FEV1 fell by more than 20% of its baselinel value, or until the highest concentration was reached. The largest value of triplicate FEV1 measurements at 30, 90, or 180 seconds after each inhalation was used in the analysis. If the PC20 was less than 16 mg/mL, a subject was considered to have AHR to methacholine.
Statistical analyses
All data were analyzed using SPSS version 7.5 for Windows. Each biochemical assay was repeated at least twice. Data are expressed as mean±standard error of the mean (SEM). Statistical analysis was performed using Student's t-test, the Mann-Whitney U test, and the chi-square test. In the multivariate analysis, logistic regression was performed with a stepwise selection method using p<0.05 as the criterion for entry into the model. Atopy and asthma symptoms were treated as binary outcome variables in the logistic regression analysis. A p-value of <0.05 was considered significant.
RESULTS
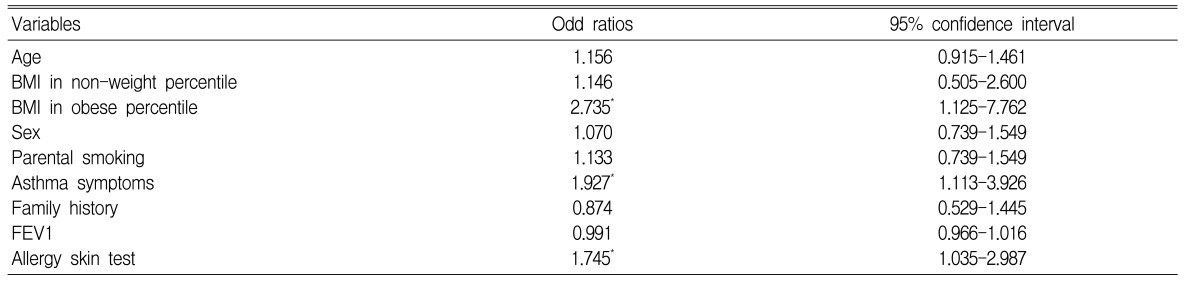
The characteristics of the schoolchildren are shown in Table 1. The mean BMI was 17.1±0.09 kg/m2 (range, 10.8-29.9). The prevalence of AHR was 42.7% (285/667). The sensitization rate (skin prick test; allergen/histamine ≥3+) to common inhalant allergens was 30.3% (202/667). The mean BMI of boys was not different from that of girls (17.09±0.13 kg/m2 vs. 17.12±0.12 kg/m2, respectively). The PC20 in children with BMIs ≥17.1 kg/m2 (6.05±0.43 mg/mL) was significantly lower than in children with BMIs <17.1 kg/m2 (7.75±0.57 mg/mL, p<0.05). The prevalence of AHR in boys was not different from that in girls (146 of 318 boys vs. 170 of 348 girls). The children were grouped by sex into percentile of BMI. The PC20 in boys was lower in the obese group than in the overweight and non-weight groups (n=9, 3.65±1.10 mg/mL vs. n = 135, 6.36±0.50 mg/mL vs. n=11, 8.38±1.82 mg/mL, respectively; p<0.05). The PC20 in girls was not different by percentile of BMI (obese, n=11, 7.72±2.74 mg/mL vs. non-weight, n=153, 6.90±0.53 mg/mL vs. overweight, n=16, 8.00±2.11 mg/mL). The PC20 in boys and girls with atopy was significantly lower than that in boys and girls without atopy (boys, 5.12±0.74 mg/mL with atopy vs. 7.09±0.59 mg/mL without atopy, p<0.01; girls, 5.05±0.81 mg/mL with atopy vs. 8.08±0.65 mg/mL without atopy, p<0.01). In a multiple logistic regression model that included all of the children, adjusted for age, sex, smoking status of parents, FEV1, and family history, independent associations with AHR were seen with BMI (OR=2.735; 95% CI=1.125-7.762, p=0.045), asthma symptoms (OR=1.927; 95% CI=1.113-3.926, p=0.012), and atopy (OR=1.745; 95% CI=1.035-2.987, p=0.018) (Table 2). In a multiple logistic regression model that included only boys and was adjusted for BMI, age, sex, FEV1, atopy, and family history, independent associations with AHR were seen with smoking status of parents (OR=1.999; 95% CI=1.109-3.603, p=0.021) and asthma symptoms (OR=1.325; 95% CI=0.789-1.764, p=0.027) (Table 3).
DISCUSSION
This epidemiological survey showed that BMI, asthma symptoms, and atopy were associated with AHR. Boys who were obese had severe AHR, and the status of parents' smoking was an independent risk factor for AHR in boys. These results indicate that BMI may contribute to the development of AHR. Furthermore, specific interventions aimed at preventing excessive weight gain and smoking exposure in schoolchildren, especially in boys, may be a valuable step in preventing the development of asthma.
The marked increase in obesity over the last 20 years might be contributing to the concomitant increase in asthma prevalence. The usefulness of BMI as an indicator of adiposity in children and adolescents has been demonstrated17, 18). The prevalence of symptoms of atopy and rhinitis in girls6) was greater in those in the highest BMI quintile. The BMI-rhinitis association was dependent upon the BMI-atopy relationship. On the other hand, wheezing and AHR were significantly less common in girls in the lowest BMI quintile, and remained so after adjusting for atopy and other potential risk factors for bronchial hyperresponsiveness. Asthma induces a decrease in energy intake that does not result in a concomittant decrease in body weight. This suggests that there is a reduction in energy expenditure in asthma, perhaps caused by a disease-induced limitation of physical activity19). A number of studies over the last few decades have found greater prevalence of asthma in higher socioeconomic groups20, 21). In the Netherlands22), 793 middle-aged males were followed from 1960-1985. The intake of linoleic acid was positively associated with the risk of developing chronic nonspecific lung disease.
We found that BMI was associated with AHR in all of the children in a multiple logistic regression model that included atopy, age, sex, FEV1, family history, and smoking status of parents. This observation is consistent with that of other authors4), who have shown that height-adjusted levels of airway lung function are strongly correlated with BMI in schoolchildren of both sexes. Obesity is not associated with asthma in healthy Korean children23). Differences between our study and Kang's reports23) may be due to population recruitment including: doctor's diagnosis, questionnaires, and epidemiologic studies. In contrast to the findings of a previous epidemiological study8-11), boys in the obese BMI percentiles have lower PC20, suggesting that BMI may contribute to AHR. This observation suggests that factors other than female hormones, directly or indirectly involved in the putative causal pathway relating obesity to asthma, may explain the development of asthma. Further studies are needed to clarify the role of sex in AHR.
Factors leading to an increase in asthma and allergy have not been identified, although exposures related to general changes in the domestic environment are likely to be involved. Environmental factors play an important role in the development and manifestation of allergic conditions in genetically predisposed subjects24). In this study we observed that the prevalence of atopy in schoolchildren was 30.3% and that atopy was an independent risk factor in AHR. This indicates that atopy plays an important role in the development of asthma in schoolchildren.
Maternal smoking during pregnancy is independently associated with decreased lung function in school-age children25). Exposure to environmental tobacco smoke was associated with a decline in peak flow and an increase in respiratory symptoms, and the use of bronchodilators in asthmatic children26). Passive smoking is a major cause of respiratory morbidity in children27). In this study, parental smoking status was a risk factor for AHR, suggesting that passive smoking may be an causal factor of AHR. Preventing exposure to smoking is necessary to prevent asthma-related symptoms.
In conclusion, this study showed that BMI was associated with AHR. Obese boys had severe AHR, and parental smoking status was a risk factor for AHR in boys. These results suggest that monitoring BMI and exposure to environmental smoke could play an important role in preventing the development of asthma. Further follow-up studies are needed to evaluate the role of body weight and sex in bronchial asthma in schoolchildren.
Notes
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ3-PG6-01GN04-003)