The Effects of Methylene Blue on Hemodynamic Parameters and Cytokine Levels in Refractory Septic Shock
Article information
Abstract
Background/Aims
Endogenous nitric oxide (NO) induces the peripheral vasodilation via the activation of guanylate cyclase in patients with septic shock. The purpose of this study was to assess the acute effects of methylene blue (MB), which is an inhibitor of guanylate cyclase, on the hemodynamics and on the production of pro-inflammatory cytokines and nitric oxide (NO) in patients with refractory septic shock.
Methods
Twenty consecutive patients with refractory septic shock, which was defined as shock refractory to a dopamine infusion of more than 20 µg/kg/min with the appropriate use of antibiotics and adequate volume replacement, received MB infusion of 1 mg/kg intravenously. The hemodynamic and respiratory variables were measured at baseline, 30, 60 and 120 min after an infusion of MB (1 mg/kg). The blood levels of NO, IL-1, IL-10 and TNF-α were measured at baseline, 30 and 120 min after MB infusion.
Results
The administration of MB induced an increase in the systemic vascular resistance (SVR) that resulted in an increase of the mean arterial pressure (MAP) in patients with refractory septic shock, and this was without a decrease in cardiac output. The administered MB induced an increase in pulmonary vascular resistance (PVR) that resulted in an increase of pulmonary arterial pressure (PAP), without any deterioration of gas exchange. However, the increases in SVR and PVR were not associated with the alteration of endogenous production of NO, IL-1, IL-10 and TNF-α.
Conclusion
MB transiently elevated the MAP by increasing the SVR without altering the endogenous productions of NO, IL-1, IL-10 and TNF-α during the study period in patients with refractory septic shock.
INTRODUCTION
The hemodynamics of septic shock are characterized by peripheral arteriolar vasodilation, and this condition leads to a hyperdynamic state with low systemic vascular resistance, high cardiac output, hypotension and inadequate tissue perfusion1, 2). In clinical practice, there is no optimal therapeutic option for septic shock patients who are unresponsive to adequate fluid resuscitation and inotropics.
Nitric oxide (NO) has been shown to play a key role in the pathogenesis of septic shock3-5). Endotoxin and various cytokines such as interleukin (IL)-1, and tumor necrosis factor (TNF)-α in the circulation of septic shock patients stimulate the synthesis of NO by activating inducible nitric oxide synthase (iNOS)3, 6-8). The increased NO stimulates the soluble guanyl cyclase enzyme in the vascular smooth muscle, and this leads to the production of cyclic GMP that in turn results in endothelial smooth muscle relaxation. The final consequence of all of this is vasodilatation and arterial hypotension9).
Methylene blue (MB), is an inhibitor of the guanylate cyclase enzyme, and it has been studied as a potential vasopressor in septic shock. In fact, the administration of MB has been reported to transiently increase blood pressure in patients with septic shock10-15). Moreover, when MB was continuously infused into patients with septic shock, it counteracted myocardial depression, maintained oxygen transport and reduced adrenergic support compared with conventional treatment alone16,17). As for the underlying mechanisms of its hemodynamic effect on septic shock, MB has been suggested not only to hamper the action of NO by the inhibition of soluble guanyl cyclase of the vascular smooth muscle, but also to inhibit the production of NO18,19).
Considering that endogenous NO stimulates the expression of pro-inflammatory cytokines through the NF-kB pathway20-22), MB may also affect the endogenous production of the cytokines. However, it is not known whether the transient increase in blood pressure observed with the administration of MB is associated with the production of pro-inflammatory cytokines. Moreover, the acute hemodynamic effect of MB in septic shock patients who are unresponsive to the usual treatment has not been well reported on. We hypothesized that MB would increase the blood pressure by attenuating the synthesis of NO, IL-1 and TNF-α along with blocking the NO-induced vasodilation via guanylate cyclase inhibition in septic shock. The aims of this study were to assess the acute effects of MB on the hemodynamics and on the production of NO and pro-inflammatory cytokines in patients with refractory septic shock.
MATERIALS AND METHODS
This study was approved by the institutional Ethics Committee of Asan Medical Center. An informed consent was obtained from each of the patients or from their next of kin.
Patients
We enrolled 20 consecutive critically ill adult patients with refractory septic shock into the study. They were admitted to the medical intensive care unit of Asan Medical Center from May 1, 2000 to May 1, 2001. Septic shock was defined as sepsis with hypotension (systolic blood pressure of less than 90 mm Hg or a decrease of at least 40 mm Hg from the patient's usual blood pressure) and there were signs of tissue hypoperfusion such as oliguia, mental confusion and an increased blood lactate concentration (>2 mmol/L) despite of adequate fluid resuscitation and antibiotics. Refractory septic shock was arbitrary defined as a hypotension that was refractory to adequate fluid resuscitation as determined by a pulmonary artery occlusion pressure of greater than 12 mmHg at least for an hour after more than 1 h of continuous norepinephrine or dopamine infusion equal to or more than 0.1 or 20 µg/kg/min, respectively. The administered vasopressors were continually infused at the same rate, and other vasopressors were not added throughout the study period.
Each patient was equipped with a radial arterial line and a pulmonary artery balloon-tip catheter (Swan-Ganz catheter, Baxter Edwards Critical-Care, Irvine, CA), and each patient was intravenously infused with 1 mg/kg of MB diluted in 100 ml of saline via a central venous catheter over a period of 15 min.
Measurement of Hemodynamics
We measured the heart rate, arterial pressure, pulmonary arterial pressure, right atrial pressure, pulmonary artery occlusion pressure and cardiac output at baseline and at 30, 60 and 120 min after the infusion of MB. The cardiac output was measured by the thermodilution technique, and it expressed as the mean of the values recorded after each of 3 to 5 injections of saline. We calculated cardiac index (CI), stroke volume index (SVI), systemic (SVRI) and pulmonary (PVRI) vascular resistance index, left ventricular stroke work index (LVSWI) and right ventricular stroke work index (RVSWI) according to the standard formulas. We also measured the arterial plasma lactate concentration as a global index of tissue perfusion, and we did so at the same time points that we measured the other hemodynamic parameters. We defined the hemodynamic response to MB infusion as an acute 10% increase of MAP from the baseline.
Measurement of respiratory parameters
Arterial and mixed venous blood samples were obtained at baseline, 30, 60 and 120 min after the infusion of MB. The ratio of PaO2 to FiO2, (A-a)DO2, oxygen delivery, oxygen consumption, the oxygen extraction ratio and the pulmonary shunt fraction were also derived.
Measurement of NO and Cytokines
We measured the blood levels of NO, IL-1, IL-10 and TNF-α at baseline, 30 and 120 min after MB infusion. The IL-1, IL-10 and TNF-α levels were measured by an enzyme-linked immunosorbent assay (ELISA) by using commercially available kits (R&D Systems, Minneapolis, MN). Because NO is rapidly broken down to nitrite and nitrate, we measured the nitrite level instead of NO by an ELISA method using the commercially available kits (R&D Systems, Minneapolis, MN). Blood samples of age-matched normal healthy people were drawn to measure and compare the cytokines of healthy controls. For IL-1, the intra-assay and interassay coefficient variation of IL-1, IL-10, TNF-α, and nitrite were 3.8~5.2% and 4.8~9.1%, 2.8~6.5% and 5.8~9.2%, 2.8~4.8% and 4.0~6.1%, and 6.8~9.5% and 5.0~8.1%, respectively.
Statistics
Data are expressed as means±SD. One sample t-test, repeated measures ANOVA, Wilcoxon rank sum test, Kruskal-Wallis one way ANOVA, Fisher's exact test and correlation analysis were used as the statistical methods. A p<0.05 was considered as statistically significant.
RESULTS
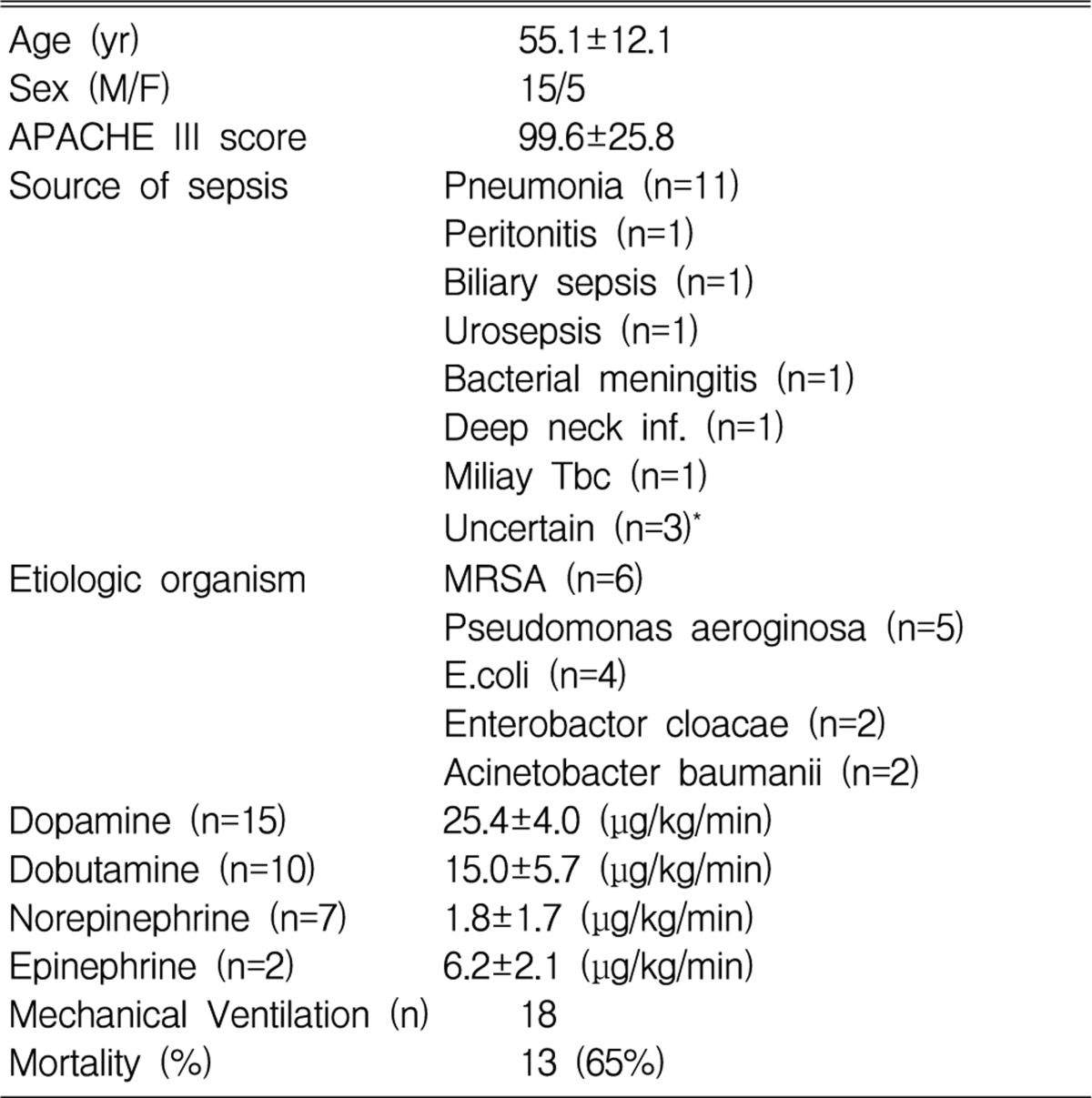
Clinical characteristics of patients (Table 1)
Eighteen patients (90%) received mechanical ventilatory support due to respiratory failure. Thirteen of the patients died, which represented a 65% mortality rate. In the survival group, the initial mean APACHE III score (81.4 vs. 109.4, p<0.05) and lactate level (3.6 mmol/L vs. 5.8 mmol/L, p<0.05) were significantly lower than those of the nonsurvival group.
Hemodynamic effects of MB (Figure 1, Figure 2)

Changes in mean arterial pressure (MAP) after the infusion of MB (mean±SEM, n=20 in each group) in refractory septic shock patients. MAP significantly increased from baseline at 30, 60 and 120 minutes.
*p<0.01 vs. baseline **p<0.05 vs. baseline

Changes in mean pulmonary arterial pressure (MPAP) after the infusion of MB (mean ±SEM, n=20 in each group) in refractory septic shock patients. MPAP significantly increased from baseline at 30, 60 and 120 minutes.
*p<0.01 vs. baseline **p<0.05 vs. baseline
The administration of MB significantly increased the MAP from 73±11 mmHg at baseline to 83±17 mmHg at 30 min (p<0.01), to 83±17 mmHg at 60 min (p<0.01), and to 80±16 mm Hg at 120 min (p<0.05) (Figure 1). The arterial pressure returned to its baseline level within 2-4 h. The mean pulmonary artery pressure (MPAP) was also significantly increased at 30 (28±7 mmHg, p<0.01), 60 (27±5 mmHg, p<0.05), and 120 min (27±5 mmHg, p<0.05) after MB infusion compared with its baseline (25±5 mmHg, Figure 2). The SVRI was increased significantly at 30 (1135±480 dyne sec/cm5/m2, p<0.05), 60 (1153±494 dyne sec/cm5/m2, p<0.05), and 120 min (1195±538 dyne sec/cm5/m2, p<0.05) after MB infusion compared with its baseline level (1011±358 dyne sec/cm5/m2). The elevated MAP returned to its baseline level within 2-4 h after MB infusion. We tried continuous MB infusion in two patients, but the continuous MB infusion could not sustain the elevated MAP in our patients (data not shown).
The PVRI was also increased significantly at 30 (200±102 dyne sec/cm5/m2, p<0.05), 60 (204±138 dyne · sec/cm5/m2, p<0.05), and 120 min (191±95 dyne sec/cm5/m2, p<0.05) after MB infusion compared with its baseline (169±93 dyne sec/cm5/m2). The PVRI change after MB infusion was also correlated with the change of the MPAP at 30 min (Pearson's correlation coefficient; r=0.49, p<0.05). The left ventricular stroke work index (LVSWI) was increased at 30 (38±18 g · m/beat/m2, p<0.05) and 60 min (38±19 g · m/beat/m2, p<0.05) after MB infusion compared with its baseline (31±12 g · m/beat/m2). The right ventricular stroke work index (RVSWI) increased at 30 min after the infusion of MB (from 8.0±4.2 to 9.6±5.8 g · m/beat/m2, p<0.05). There were no changes in heart rate, cardiac filling pressure, cardiac index and pulmonary artery occlusion pressure after an infusion of MB (data not shown).
Changes in serum lactate level by MB infusion
Arterial lactate concentration was decreased significantly at 30 (4.90±4.31 mmol/L, p<0.05), 60 (4.84±4.22, p<0.05), and 120 min (4.76±4.57 mmol/L, p<0.05) after MB infusion compared with its baseline level (5.52±4.68 mmol/L).
Respiratory effects of MB
The oxygen delivery, oxygen consumption and oxygen extraction ratio remained unchanged by MB infusion (data not shown).
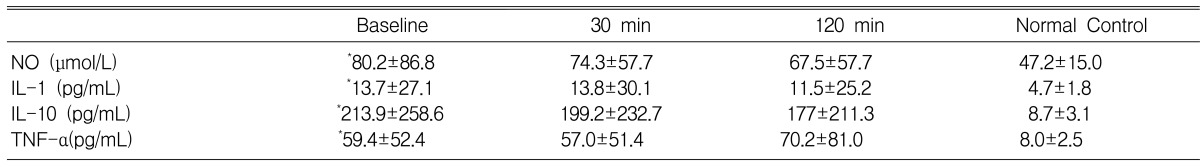
Effect of MB on the endogenous production of NO and Cytokines (Table 2)
The basal levels of the cytokines in the patients were significantly higher than those of the normal controls. However, an infusion of MB did not change the blood levels of Nitrite, IL-1, IL-10 and TNF-α during the study period.
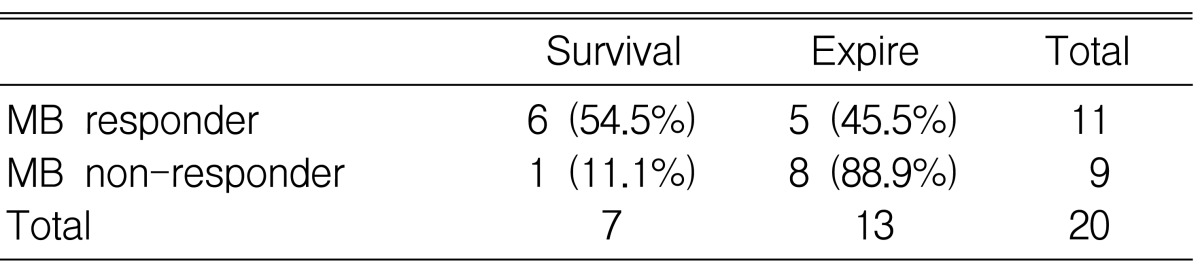
The association of MB response with survival (Table 3)
Among the 11 MB responders, 6 patients survived and 5 patients died. However, only 1 patient survived among 9 MB non-responders. However, the difference was not statistically significant (p=0.07).
DISCUSSION
MB induced an increase in the SVR resulting in the transient increase of the MAP in our patients with refractory septic shock, and this was without a decrease in the cardiac index. MB also induced an increase in the PVR, which resulted in an increase of the PAP. However, there was no deterioration of gas exchange. These findings were consistent with previous reports10-15). MB increased the left ventricular stroke work index without changing the cardiac filling pressure and with increasing the systemic vascular resistance. The myocardial function could be improved by MB because MB increased the sensitivity of the cardiovascular system to catecholamines by inhibiting the excessive production of cGMP23). If these effects could be maintained by MB infusion, MB could be a treatment option to the usual clinical practices that are employed for salvaging refractory septic shock patients. However, the effect of a single infusion of MB was not sustained in our patients for more than 4 h. Moreover, we could not maintain the elevated MAP with continuous MB infusion in two of our patients. Preiser et al. showed that the hemodynamic effects of MB were achieved with repeated does (4 mg/kg)10). Mikhail et al. demonstrated that continuously infused MB improved the cardiovascular function and reduced the requirement for adrenergic support16). A possible explanation for these different results might be the difference in the severity of the study patients. Those previous studies included septic shock patients who were unresponsive to more than 0.05 or 5 µg/kg/min of norepinephrine or dopamine, respectively. In our study, we included septic shock patients who were unresponsive to more than 0.1 or 20 µg/kg/min of norepinephrine or dopamine, respectively.
It is well known that NO is a causative molecule for the peripheral vasodilation observed in septic shock. The endotoxin and various pro-inflammatory cytokines, such as IL-1 and TNF-α, in the circulation of septic shock patients stimulate the synthesis of NO by activating inducible nitric oxide synthase (iNOS)3,6-8). On contrary, NO, which is an important endogenous regulatory molecule, has been implicated in both the pro-inflammatory and anti-inflammatory processes20). The enhanced NO generation may play an autoregulatory role in amplifying the synthesis of pro-inflammatory cytokines through the activation of NF-kB24). Considering these results, MB might alter the endogenous production of the pro/anti-inflammatory cytokines. In the research literature, MB reduced the endotoxin-induced TNF-α synthesis in Kupffer cells25), and MB decreased the plasma levels of the stable NO metabolites, nitrites and nitrates, as well as cGMP19). To determine whether the MB-induced increase in the SVR was associated with the endogenous production of pro/anti-inflammatory cytokines in septic shock patients, we assessed the acute changes of cytokines in the patients' blood after a single infusion of MB. We found that a single infusion of MB did not affect the endogenous production of IL-1, IL-10 and TNF-α during the study period, although the TNF-α and IL-10 concentrations in septic shock patients were 7 and 20 times higher, respectively, in the septic shock patients than in the normal volunteers. These results are consistent with Memis and colleagues' report in which MB infusion was shown not to change the cytokine levels or the clinical outcome in severe sepsis26). Memis measured the plasma levels of cytokines at baseline (15 min before start of the study), at immediately after MB infusion and at 24 and 48 h after MB infusion. We measured the blood level of the cytokines only at 2 h after MB infusion because such confounding factors as the underlying disease progression can affect the blood levels of the cytokines. In addition, TNF-α levels are known to rise rapidly after the injection of bacteria or endotoxin; in the animal models of shock and gram-negative sepsis, they reach peak levels at 60~90 min and then decrease 27). The IL-1 levels rise slowly and peak at 180 min28). Similar kinetics have been observed in human subjects who were injected with endotoxin29). We could not exclude the possibility of the late effect of MB on the endogenous production of the cytokines. However, Mikhail et al. noted that TNF-α and IL-6 levels remained unchanged16) after 24 h of MB treatment in their endotoxemic sheep model. Instead, the MB improvement of the cardiopulmonary function was associated with a reduction of the arachidonic acid metabolites thromboxane B2 and prostaglandin F1α30). MB seems to block prostacyclin production by a mechanism that is independent of inhibition of soluble guanyl cyclase31). Since cytokine levels rise and fall with different time courses in response to an inflammatory insult, further studies are needed about the time frame after MB infusion.
MB is known to generate superoxide anions, and this could antagonize the action of nitric oxide-induced vasodilatation by decreasing the available nitric oxide32,33). We found that a single infusion of MB did not decrease the endogenous production of NO. This result differs with a previous report that showed MB's inhibitory effect on the production of NO19). However, in animal models of sepsis induced by bowel perforation, the plasma NO level decreased after NOS inhibitor, but the plasma and urine NO levels were not changed after 4 h of MB administration34), and this is a finding consistent with our study. Martin et al. showed that MB is an inhibitor of the soluble guanylate cyclase activity that counteracts the effects of nitric oxide and other nitrovasodilators in the endothelium and vascular smooth muscle35). MB prevents the endotoxin- or IL-1-induced vasorelaxation by abolishing guanosine 3' and 5'-cyclic monophosphate production and MB reverses the endotoxin induced or cytokines induced hypotension and myocardial depression23,36). Our result supports the concept that MB exerts its antagonistic effect on NO by inhibiting the soluble guanyl cyclase without altering the NO production. There is another concern that although methylene blue may be able to reverse hypotension, it may not be able to improve organ blood flow and oxygen delivery37). Yet we found that the arterial lactate concentration was significantly decreased 30 min after MB infusion compared with its baseline, which suggests there was better tissue oxygenation.
Six patients among 11 MB responders and only 1 patient among 9 MB non-responders survived, suggesting a possible prognostic indicator of a single MB infusion. However, large randomized controlled studies are required to determine whether the initial hemodynamic response to MB infusion could be a prognostic index for patients with septic shock. The limitations of our study are the small number of patients and the absence of a control group to assess the effect of a similar volume of isotonic saline. In addition, we did not check the level of cGMP and the guanylate cyclase activity.
In conclusion, although a single administration of MB transiently elevated the MAP by increasing the SVR, endogenous productions of NO, IL-1, IL-10 and TNF-α were not altered during the study period in the patients with refractory septic shock. The acute vasopressor effect of MB seems to be mainly due to inhibition of guanyate cyclase activity. Further studies are required to determine whether MB could be an alternative to increasing vasopressor support for patients with refractory septic shock.
Notes
This study was supported by Asan Life Science Institute Grant No 2001-113.