Treatment Outcome of Adult Acute Lymphocytic Leukemia with VPD(L) Regimen: analysis of Prognostic Factors
Article information
Abstract
Background
Because of the relative paucity of data regarding the clinical outcome in adult patients with acute lymphocytic leukemia (ALL) in Korea, we analyzed clinical courses in adult ALL patients treated with VPD (L) regimen (vincristine, prednisolone, daunorubicin, L-asparaginase) at the Seoul National University Hospital, and evaluated prognostic factors influencing the outcome.
Methods
Patients with ALL newly diagnosed between October 1994 and June 2000 at our hospital were analyzed retrospectively. Fifty-three patients were evaluable. Induction chemotherapy consisted of VPD with (46 cases) or without L-asparaginase (7 cases). After complete remission (CR), consolidation therapy, CNS prophylaxis and maintenance chemotherapy were administered.
Results
Ages ranged from 16 to 67 (median 30). CR rate was 86.8% (46/53) and no significant prognostic factor was found for the CR rate. With a median follow-up time of 27.2 months (range 12.9–83.0 months) in living patients, the median overall survival (OS) for all cases was 16.7 months (13.4–20.1 months, 95% C.I.) and the estimated 4-year OS rate was 25.4%±8.9%. The median relapse-free survival (RFS) was 12.2 months (8.4–16.0 months, 95% C.I.), and 3-year RFS rate was 29.9%±10.2%. Poor prognostic factors for OS were Ph chromosome (p=0.005) and T-cell immunophenotype (p=0.03). For RFS they were Ph chromosome (p=0.01) and the presence of a mediastinal mass (p=0.03).
Conclusion
Despite an initial excellent response to the VPD (L) regimen, newer therapeutic strategies, including more intensive postremission therapies, are urgently needed because of the high relapse rate. Future therapeutic approaches need to be stratified according to several prognostic factors.
INTRODUCTION
Although acute lymphocytic leukemia (ALL) is the most common leukemia in childhood, it accounts for only about 20% of acute leukemia in adults. Significant progress has been made during the past 30 years in the treatment of both childhood and adult ALL, but in adults, ALL treatment results remain inferior to those in childhood. In children with ALL, complete remission (CR) rates are well over 90%, and long-term disease-free survival is up to 70 to 80%1). In contrast, in adults, although the current CR rate is 70–90%, only 30 to 40% of the patients can expect a cure2).
The drugs most often used for induction treatment in adult ALL are prednisone (P), vincristine (V), an anthracycline, and L-asparaginase (L). The combination of V and P alone produces CR rates of approximately 36% to 67% in adult3). However, when an anthracycline such as adriamycin (Ad) or daunorubicin (D) is added, the CR rate increases to 72% to 92% (Ad) or 72 to 89% (D)4) and the median remission duration increased to 17 to 18 months5). Combining cyclophosphamide, L-asparaginase, or cytarabine with vincristine- anthracycline-corticosteroid induction regimen was found not to improve results6–8).
In Korea, we previously investigated clinical results of VPL induction therapy and reported a CR rate of 72.2% and a median remission duration of 14.3 months9). Im et al10) reported a CR rate of 83.3% for VPDL induction therapy. Several other studies in Korea have shown similar CR rates ranging from 66.7% to 88.0%11–16).
In this study, we analyzed clinical courses and prognostic factors affecting the clinical outcome in adult ALL patients treated with VPD (L) regimen.
MATERIALS AND METHODS
1. Patient Eligibility
The medical records of 62 adult ALL patients older than 16 years were reviewed. All patients had been newly diagnosed between October 1994 and June 2000 at the Seoul National University Hospital. Patients had their diagnoses confirmed by morphologic studies of bone marrow aspiration and biopsy, according to the French-American-British (FAB) criteria17). In addition, cytogenetic studies, immunophenotypic studies and molecular analysis by RT-PCR (reverse transcriptase-polymerase chain reaction) for the bcr/abl fusion gene were performed. Among 62 patients, 9 patients were excluded because of death before treatment (1 case), treatment refusal (3 cases), an age of more than 70 years (1 case), a previous history of non-Hodgkin’s lymphoma (2 cases) and induction chemotherapy with other than VPD (L) (2 cases). Finally, 53 patients were available for the study.
2. Treatment
1) Induction therapy
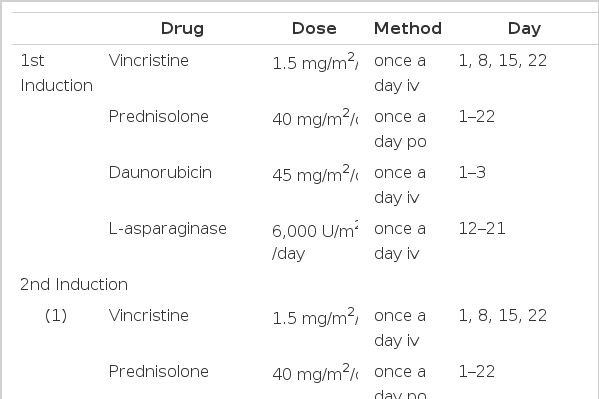
Induction therapy consisted of vincristine (V), prednisolone (P) and daunorubicin (D) with or without L-asparaginase (L). Patients received vincristine, 1.5 mg/m2/day (maximum 2 mg) i.v., on days 1, 8, 15 and 22 and prednisolone, 40 mg/m2/day (maximum 60 mg) p.o., on days 1 through 22. The dose of prednisolone was then tapered over six days and discontinued. Daunorubicin, 45 mg/m2/day i.v., was administered on days 1 through 3 and L-asparaginase, 6,000 U/m2/day i.v., was given on days 12 to 21. Forty-six of the 53 patients received the VPDL regimen and seven patients received the VPD regimen due to elevated liver enzymes. If successful remission induction was not achieved after the 1st induction therapy, the 2nd induction therapy was administered. This consisted of various regimens, including VPD plus cyclophosphamide (4 cases) or VPD (2 cases) or VPDL (1 case) or mitoxantrone (M) plus Ara-C (A) (1 case) or Ara-C plus cyclophosphamide plus mercaptopurine (1 case) (Table 1).
2) Consolidation therapy
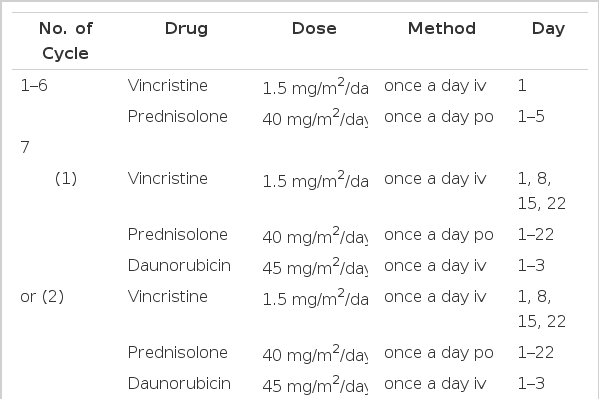
Patients achieving CR received consolidation therapy. Consolidation therapy consisted of six monthly cycles of VP (vincristine, 1.5 mg/m2/day i.v., day 1 and prednisolone, 40 mg/m2/day p.o., days 1 to 5) plus, thereafter, one cycle of VPD (17 cases) or VPDL (2 cases) or MA (5 cases). Five patients with an HLA-identical sibling received allogeneic bone marrow transplantation (alloBMT) (Table 2).
3) Maintenance therapy
Following CR, maintenance therapy was administered before and after consolidation therapy for 24 months. This involved a combination of methotrexate 20 mg/m2 p.o., administered weekly and 6-mercaptopurine 75 mg/m2/day p.o., administered daily.
4) CNS prophylaxis
Cranial irradiation (2,400 cGy) and intrathecal (IT) methotrexate 12 mg/m2 biweekly (total of 5 doses) were administered after CR.
3. Response criteria
Patients were considered to be CR if the proportion of blasts in their bone marrow was less than 5%, the bone marrow cellularity was normal (>25%) and the peripheral blood cell count was adequate (absolute neutrophil count (ANC), more than 1.5×109/L; platelet count, more than 100×109/L) for at least 1 month. All other responses were defined as failures and categorized as (1) early death, if patients died before response to therapy could be assessed or (2) primary refractory disease if they survived the 2nd remission induction with persistent leukemia.
Overall survival (OS) was calculated from the date of diagnosis until death or. the last follow-up date. Relapse-free survival (RFS) duration was calculated for CR patients from the date of CR until documented relapse, death or the last follow-up date in remission. The time to achievement of CR was determined from the initiation of induction chemotherapy until the first documentation of remission in tte bone marrow.
4. Statistical analysis
The prognostic significances of various clinical and hematopathological characteristics were assessed with respect to the CR rate, RFS and OS. The different response rates were compared with the subgroup characteristics, and analyzed using chi-square and Fisher’s exact tests. RFS and OS curves were plotted by the Kaplan and Meier method and were compared by the log-rank test. The Cox proportional hazards model was used to analyze the association between the aforementioned factors, the survival time and the duration of RFS. Variables that attained a univariate p value of .20 or less were considered in the variable selection process for regression analyses. Reported p values were nominal two-sided values, unless otherwise stated. p value of .05 or less was considered as statistically significant.
RESULTS
1. Patient Characteristics
Clinical characteristics and hematopathology of the eligible 53 patients are summarized in Table 3. The 53 eligible patients ranged in age from 16 to 67 years (median 30 years). Thirty patients (56.6%) were male and 23 (43.4%) were female. The initial WBC counts ranged from 0.79 to 368×109/L (median 18.3×109/L).
Only two patients (4.0%) presented with symptomatic CNS disease at diagnosis or during induction. None had gonadal involvement at diagnosis.
The frequency of immunologic subtypes in the 51 typed patients was B-cell precursor in 36 (70.6%), mature B-cell in 3 (5.9%) and T-cell in 12 (23.5%). No differences were found with respect to age, WBC count, the proportion of Philadelphia (Ph) chromosome, or the percentage of peripheral blasts according to immunophenotype. However, there was a male predominance (83.0% in T-cell ALL vs 50.0% in B-cell precursor ALL, p=0.05) and a high proportion of patients with mediastinal mass (41.7% in T-cell ALL vs 0% in B-cell precursor ALL, p<0.001) in T-cell ALL.
Chromosomal analysis was performed in 43 cases, but in 12 cases the specimens were inadequate due to insufficient mitosis. Therefore, 31 cases (59.0% of the entire population) were evaluable. An abnormal karyotype pattern was noted in 17 patients (17/31, 54.8%) leaving 14 (14/31, 45.2%) patients free of any chromosomal abnormality. Ph chromosome was detected in 6 patients based on the presence of t(9;22) (q34;q11). Molecular study for the bcr-abl fusion gene, using RT-PCR found 3 additional cases (2 cases were Ph-negative by conventional cytogenetic analysis and in one case cytogenetic result was not available). Among the 22 cases in which chromosome study was not performed or inadequate, 14 cases underwent a molecular study. Therefore, in total, 45 cases were evaluable for Ph/bcr-abl by chromosome study and/or molecular study, and 9 cases (9/45, 20.0%) had Ph/bcr-abl+ ALL.
2. Treatment Results
Forty-six of 53 patients (86.8%) treated with induction chemotherapy achieved CR; 40 of them after the 1st induction therapy (75.5%). Six additional CRs were achieved among the 9 patients who received the 2nd induction therapy. Two patients (3.8%) were considered as refractory ALL and 5 patients (9.4%) died of infection. The median time to achieve CR was 29 days (range 20–123 days).
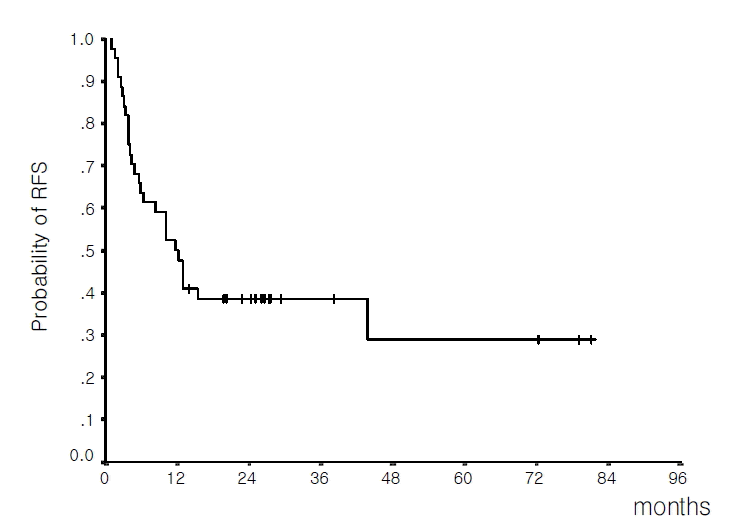
With a median follow-up time of 27.2 months (range 12.9–83.0 months) in living patients, the median OS for all cases was 16.7 months (13.4–20.1 months, 95% C.I.) and the estimated 4-year survival rate was 25.4%±8.9% (Figure 1). The median OS for patients who achieved CR was 19.0 months (15.3–22.7 months, 95% C.I.) and the estimated 4-year survival rate was 29.3%±10.1%. In contrast, in patients who failed to achieve CR, the median survival was 1.5 months (0.7–2.3 months, 95% C.I.).
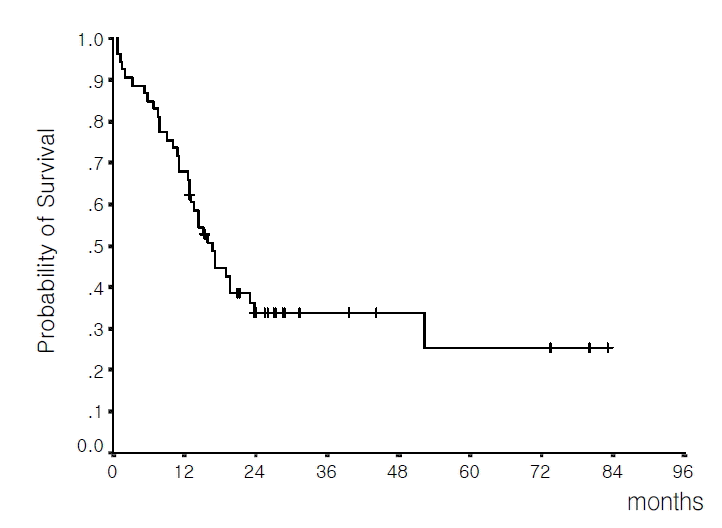
Overall survival of the 53 evaluable patients (median 16.7 months; estimated 4-year survival rate 25.4%).
Median RFS duration for the 46 patients who achieved CR was 12.2 months (8.4–16.0 months, 95% C.I.); the probability of being in CR at 3 years was 29.9%±10.2% (Figure 2).
3. Relapses
At the time of analysis, 28 (60.9%) of 46 patients who had achieved CR relapsed, 16 (34.8%) remained in remission and 2 (4.3%) were lost to follow-up. Six relapses (21.4%) occurred within 3 months of the date of CR, 10 (35.7%) from 3 to 6 months, 6 (21.4%) between 6 and 12 months, 5 (17.9%) in the second year, and 1 (3.6%) after 2 years. The sites of relapse were the bone marrow (n=24, 85.7%), the CNS (n=2, 7.1%), combined marrow and CNS (n=1, 3.6%), and combined marrow and stomach (n=1, 3.6%). All the CNS relapses were of the T-cell immunophenotype. Differences in the patterns of relapse according to immunophenotypes were not analyzed further because of the small sample size. There were no documented testicular relapses among the male patients.
4. Prognostic Factors
2) Overall survival duration
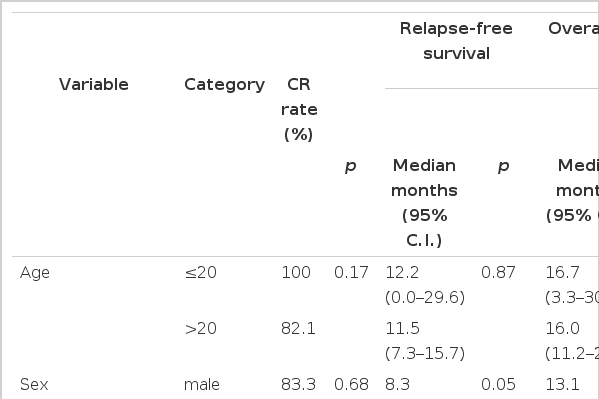
By univariate analysis, potential poor prognostic factors for OS (p<0.2) were; male gender (p=0.04), the need for a 2nd induction to achieve CR (i.e. delayed time to CR) (p=0.01), Ph chromosome/bcr-abl (p=0.16), LDH (p=0.09) and T-cell immunophenotype (p=0.09) (Table 4). Multivariate analysis with these variables showed two associated factors with a shorter OS: ph chromosome/bcr-abl (p=0.005) and T-cell immunophenotype (p=0.03) (Table 5).
3) Relapse-free survival
By univariate analysis potential poor prognostic factors for RFS (p<0.2) were; male gender (p=0.05), the presence of a mediastinal mass (p=0.03), the need for a 2nd induction to achieve CR (i.e. delayed time to CR) (p=0.003), Ph chromosome/bcr-abl (p=0.09) and T-cell immunophenotype (p=0.13) (Table 4). By multivariate analysis these variables, poor prognostic factors for RFS were; Ph chromosome/bcr-abl (p=0.01) and the presence of a mediastinal mass (p=0.03) (Table 5).
DISCUSSION
Significant progress has been made in the treatment of adult ALL and many previous studies have reported high CR rates (70–90%) after intensive induction chemotherapy in adult ALL. Linker et al18) reported a CR rate of 88% with VPDL induction therapy and Hoelzer et al19) reported a CR rate of 73.9%. In Korea, similar CR rates, of 66.7 to 88.0%, have been reported with various regimens9–16). In our retrospective analysis of treatment outcome in adult patients receiving the VPD (L) regimen, the CR rate after the 1st induction chemotherapy was 75.5% and the overall CR rate (including the 2nd induction chemotherapy) was 86.8%.
Although substantial improvements in the adult remission rate have been achieved, unfortunately, the majority patients relapse, usually within the first 2 years and long-term RFS does not exceed 30–40%. Linker et al18) and Hoelzer et al19) reported an RFS rate of 35% and 37%, respectively. In Korea, Kim et al9) reported an overall median survival of 9.7 months and Woo et al14) reported a median survival of 79 weeks and a median RFS of 80 weeks. In our study, a high relapse rate (60.9%) caused a short median OS of 16.7 months, a median RFS of 12.2 months, and only a small proportion of patients in long-term remission. Although direct comparisons between our study and others are difficult, because of differences in the patient characteristics, induction regimens, and variations in postremission therapies, the long-term outcomes of our study are inferior to several recent results19–22). The reason for this might be explained by the fact that relatively less intensive consolidation therapy (six cycles of VP plus one cycle of VPDL or VPD or MA) was used in our study.
The advantage of intensive postinduction therapy was demonstrated in Memorial Sloan-Kettering Cancer Center studies with protocols L-2 to L-17M23). Kantarjian et al21) used VAD induction regimen (vincristine, adriamycin, and dexa-methasone) and maintenance therapy with intensification with adriamycin, a high-dose of cytarabine, BCNU, cyclophosphamide, and etoposide and showed improved RFS results; median remission duration was 22 months and median survival 19 months. However, less favorable results have also been obtained. Several institutions tested successive and continuously intensified postremission chemotherapeutic protocols and have found no improvement in long-term survival rates4, 24–26).
It remains controversial as to how intensive consolidation or intensification treatment should be. However, to decrease the early relapse rate, future studies will have to be focused on more intensive consolidation including alloBMT.
In terms of prognostic variables, a variety of factors have been reported to be correlated with remission rate, remission duration and survival. Prognostic factors are often vary among studies; however, recent ALL trials with intensive chemotherapy have shown similar risk factors, e.g., older age-over 35, over 50, over 60 years; karyotype-t (9;22) or t (4;11); time to achieve CR-4 or 5 weeks or longer; high initial WBC count over 25×109 to 35×109/L8, 19, 22, 24).
In this analysis, Ph-positive ALL had the worse OS (p=0.005) and RFS (p=0.01) but was not associated with a lower CR rate. Eight of 9 Ph-positive ALL patients (88.9%) achieved CR. The median OS and RFS duration were, 10.8 months and 3.7 months, respectively, for Ph-positive ALL versus 17.3 months and 12.8 months for Ph-negative ALL. Two of these patients received alloBMT as postremission therapy; one relapsed (CR duration; 12.9 months) and the other remains alive at 73.5 months. Four Ph-positive patients did not receive consolidation chemotherapy because of early relapse. Although the number of cases is small, these results suggest that Ph-positive ALL should receive allogeneic stem cell transplantation for long-term survival.
The presence of a mediastinal mass suggesting large tumor burden was associated with short RFS in our study. Five of 6 patients (83.3%) with a mediastinal mass had T-cell immunophenotype. Mediastinal mass itself was found to be an independent adverse prognostic factor, irrespective of the immunosubtype.
The T-cell immunophenotype was formerly an unfavorable subset, particularly for patients with a mediastinal mass, with an RFS of 10 to 20%. Now, the CR rate is 80% to 95%, and RFS has increased to 45% to 60%18, 22, 27). Recent improvements in the outcome of patients with T-cell ALL have been attributed to the addition of cyclophosphamide and cytarabine to the induction and/or consolidation treatments. In our study, 12 T-cell ALL patients received induction with VPDL and only 6 patients (50.0%) achieved remission with the 1st induction therapy. Four patients that failed to achieve CR received a 2nd induction therapy with regimens containing cytarabine or cyclophosphamide and all except one, who died of infection during the 2nd induction, achieved remission. Therefore, T-cell ALL patients showed a total CR rate of 75.5%, a median RFS duration of 10.0 months, and a median OS of 15.2 months, which were inferior to recent results for regimens containing cytarabine or cyclophosphamide as an initial induction therapy. Also, in our analysis, although there was no significant difference in the CR rates of B-cell precursor and T-cell subtypes and their median RFSs, OS was worse in T-cell ALL than in B-cell precursor ALL. In addition, in our study only one of the T-cell ALL patients received consolidation therapy with a regimen containing cytarabine, and this may have contributed to poor outcome in T-cell ALL patients. Similarly, when investigators at lowaomitted an cytarabine-containing early consolidation phase, their T-cell patients did extremely poorly28). These results suggest that a remission-induction or consolidation program for T-cell ALL should include cytarabine or cyclophosphamide.
Delayed achievement of CR (i.e., requirement of a 2nd induction to achieve CR) had adverse prognostic effects upon RFS and OS by univariate analysis. However this effect was not substantiated by multivariate analysis. This result differs from those obtained in other studies18, 19, 24, 25), and may be explained by the small number of patients who achieved remission after the 2nd induction (n=6, 13.0% of the CR patients).
Other clinical and laboratory features that were analyzed in this study were found to have no influence on prognosis.
In summary, induction chemotherapy using the VPD (L) regimen was effective in inducing CR in adult ALL patients. However, because many ALL patients eventually relapsed, the long-term outcome was unsatisfactory. To further improve long-term prognosis, future trials may need more intensive and more immediate postremission therapies, especially in high-risk patients. In terms of the assessment of prognostic variables, although relatively small numbers were involved, we were able to identify several prognostic factors (Ph chromosome, T-cell immunophenotype, the presence of a mediastinal mass), which were related to OS and/or RFS in adult ALL patients. Future therapeutic approaches may need to be stratified according to these prognostic factors.