Expression of Vascular Endothelial Growth Factor and p53 in Pancreatic Carcinomas
Article information
Abstract
Background:
Angiogenesis has been shown to be a critical aspect of tumor growth and progression. Vascular endothelial growth factor (VEGF) has potent angiogenic activity and has been identified in a wide variety of malignancies, including pancreatic carcinoma. The tumor-suppressor gene p53 has been thought to regulate VEGF in angiogenesis. The aim of the current study was conducted to investigate the association between p53 mutation and VEGF expression and the prognostic value of these factors in pancreatic carcinoma.
Methods :
Formalin-fixed, paraffin-embedded tissue specimens were obtained from 30 patients who underwent surgery for pancreatic carcinoma. We used an immunohistochemical technique to localize VEGF and p53 in pancreatic carcinoma tissues.
Results :
Positive expression of VEGF was detected in 17 out of 30 (56.7%) tumors. Positive expression of VEGF correlated with the depth of tumor invasion (p=0.002). There was a trend towards an association between positive expression of VEGF and distant metastasis, although these associations were not statistically significant (p=0.070). p53 mutations were identified in 18 out of 30 (60.0%) tumors. However, no significant correlation was found between p53 expression and various clinicopathological parameters. The correlation between p53 mutation and VEGF expression was statistically significant (p=0.004).
Conclusion :
VEGF, a key factor for the induction of tumor-associated angiogenesis, may be involved in tumor characteristics, including tumor invasion and metastasis. And p53 mutation may be implicated in the regulation of angiogenesis through a VEGF up-regulation.
INTRODUCTION
The incidence of pancreatic carcinoma has risen steadily over the recent decades. Since pancreatic carcinoma is diagnosed at an advanced stage and because of the lack of effective therapies, the prognosis of such patients is extremely poor. Surgery being the only potential cure, the prognosis of these patients is critically determined by diagnosing early stage pancreatic carcinoma1,2).
Angiogenesis is an essential process for the primary tumor to grow and invade into the adjacent normal structures. Angiogenesis is likewise considered to permit shedding of cells from a primary tumor to distant body sites, thus facilitating the metastatic process3–5). The induction of angiogenesis by a tumor is a controlled process, influenced by angiogenic and angiostatic regulators which involve a complex interaction between tumor and endothelial cells6,7). Among the many reported angiogenic regulators, vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen and an angiogenic regulator released by a variety of tumor cells8–10). Expression of VEGF is increased in various human tumors compared with normal tissues, often correlating with tumor angiogenesis and poor prognosis11–16). Also, similar studies have shown these findings in pancreatic carcinoma17–25).
Recognition of the importance of angiogenesis for the growth and metastasis of cancers has raised fundamental questions regarding the molecular mechanisms of the angiogenic switch during tumor progression. The genetic alterations that are responsible for oncogenesis and tumor progression may underlie the ability of tumors to switch to an angiogenic phenotype6,7). Mutations of the p53 tumor suppressor gene represent one of the most common genetic alterations in human cancers and the acquisition of such defects is strongly associated with tumor progression and metastasis26–29). Recently, expression of the p53 gene has been demonstrated to be related to tumor angiogenesis30–34). A mutant p53 gene has been suggested to contribute to the angiogenic process through suppression of a strong inhibitor of angiogenesis, namely thrombospondine-1. Evidence also suggests that a mutant p53 gene is a potent stimulant of VEGF.
A few studies have indicated that angiogenesis may be regulated, in part, by function of the p53 tumor suppressor gene in human pancreatic carcinoma18,24). The aim of the current study was conducted to investigate the association between p53 mutation and VEGF expression, and the relationship between these factors and various clinicopathological parameters in pancreatic carcinoma.
MATERIALS and METHODS
Clinical Materials
The specimens of pancreatic carcinomas were obtained from 30 patients who underwent surgery from January 1998 to December 2000 at Chonnam National University Hospital, Gwangju, Korea. None of the patients had undergone chemotherapy or radiotherapy before surgery. Formalin-fixed and paraffin-embedded tissue specimens were taken from representative cancerous lesions over their greatest length and included the adjacent noncancerous area. The clinicopathological findings, including sex, age, tumor size, tumor location, histologic grade and stage, were obtained by medical records and pathologist and physician contact when necessary. The tumors were classified by stages according to the American Joint Committee on Cancer35) and by histological types according to Social et al36). This study group comprised 20 males and 10 females. The mean age was 59.8±12.0 (mean+SD) with a range from 30 to 81 years. The mean size of the tumor was 4.3±1.6 (mean±SD) with a range from 2.0 to 7.5 cm.
Immunohistochemistry
All procedures for immunohistochemical staining were done by the Micro-Probe staining system (Fisher Scientific, Pittsburgh, PA) based on capillary action37). Paraffin sections, of 5 μm in thickness with mounted probe on slides, were immunostained with anti-mouse monoclonal antibodies by the avidin-biotin peroxidase complex method37). Three serial sections of each specimen were stained. The immunostaining was considered to be positive only if the color reaction was equal in intensity and pattern for each section. Sections were deparaffinized and heated in a microwave oven for 7 minutes to retrieve the antigens. They were immersed in 0.6% hydrogen peroxide for 5 minutes to block the endogenous peroxidase activity. A polyclonal antibody against VEGF (A-20; diluted 1:100; Santa Cruz Biotechnology, Santa Cruz, Calf.) and a monoclonal mouse antihuman p53 antibody (DO-7, diluted 1:150; Dakopatts, Glostrup, Denmark) were used as primary antibodies. The primary antibodies, in the aforementioned concentrations were diluted in phosphate buffered saline supplemented with 5% normal horse serum and 1% bovine serum albumin and then incubated with tissues for 120 minutes at room temperature. Anti-mouse immunoglobulin G (Sigma, St. Louis, MO) labeled with biotin was added as a secondary antibody for the detection of primary antibodies and the samples were incubated for 10 minutes at 45°C. After multiple rinses with universal buffer, streptavidin-horseradish peroxidase detection system (Biomeda, Foster, CA) was applied for 10 minutes. As the final step, the slides were developed for 15 minutes with the enzyme substrate 3 amino-9-ethyl carbazole (AEC, Sigma, St. Louis, MO). The slides were counterstained with hematoxylin solution for 1 minute (Research Genetics, Huntsville, AL). After dehydration, the tissue was sealed with a universal mount (Research Genetics, Huntsville, AL).
Assessments of VEGF and p53 Expression
Assessment of the staining was examined by at least two independent observers without knowledge of the clinical outcomes and a high level of concordance was achieved. Staining intensity of VEGF was graded on a scale with four grades: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining, as reported previously18–20). The specimens with a score of more than 1 were regarded as positive expression, and those with a score≤1 as negative expression. Immunoreactivity of p53 was graded as follows: positive expression, ≥10% of tumor cells were stained; negative expression, no staining or <10% of tumor cells were stained.
Statistical Analysis
The correlation between VEGF, p53 expression and various clinicopathological parameters was analyzed for statistical significance by the χ2-test and Fisher’s exact probability test. The statistical software program used was Statistical Package for the Social Sciences (SPSS/PC+ 10.0, Chicago, IL). A p-value of less than 0.05 was accepted as statistically significant.
RESULTS
Expression of VEGF and p53 in pancreatic carcinoma
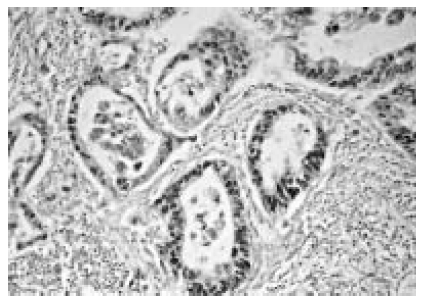
In carcinoma, positive immunostaining for VEGF was identified predominantly in the cytoplasm of the tumor cells (Figure 1). In non-cancerous tissues, islet cells were clearly stained, but acinar and ductal cells were not stained. Positive immunostaining for p53 was present intensely in the nuclei of tumor cells (Figure 2).

Immunostaining of vascular endothelial growth factor (VEGF) in pancreatic carcinoma tissues. VEGF expression was identified predominantly in the cytoplasm of the tumor cells (×200).
Correlation between VEGF and p53 expression
The positive expression of VEGF and p53 was 56.7% (17 out of 30), 60.0% (18 out of 30), respectively. The correlation between VEGF and p53 expression was statistically significant (p=0.004, Table 1).
Correlation between VEGF and p53 expression and clinicopathological parameters
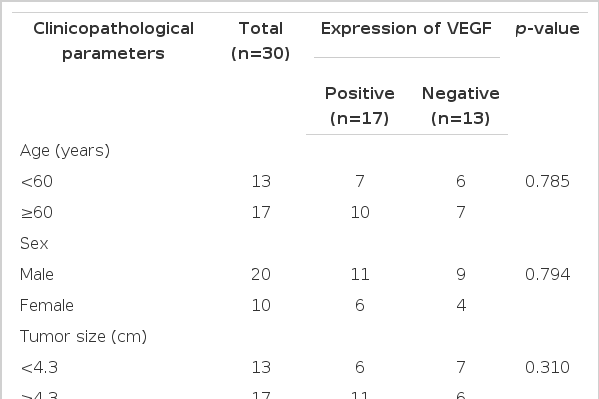
The correlation between VEGF and p53 expression and clinicopathological parameters is summarized in Table 2 and 3 respectively. Positive expression of VEGF correlated with the depth of tumor invasion (p=0.002). There was a trend towards an association between positive expression of VEGF and distant metastasis, although these associations were not statistically significant (p=0.070). VEGF expression did not correlate with patient’s age, sex, tumor size, location, histologic grade, stage or lymph node metastasis. However, no significant correlation was found between p53 expression and various clinicopathological parameters.
DISCUSSION
It has been widely accepted that tumor angiogenesis is one of the most crucial steps in tumor invasion and metastasis3–5) and many angigenic factors, such as VEGF and basic fibroblast growth factor, have been demonstrated to be involved in such biological behaviors of tumors6–10). In the current study, we have shown that positive expression of VEGF correlated with the depth of tumor invasion. Also, there was a trend towards an association between positive expression of VEGF and distant metastasis, although these associations were not statistically significant. Leda et al reported that VEGF expression was an independent prognostic factor of the various clinicopathological parameters in pancreatic carcinoma patients23). These results suggest that VEGF, a key factor for the induction of tumor-associated angiogenesis, may be involved in tumor characteristics, including tumor invasion and metastasis. However, Ellis et al reported that VEGF expression was not associated with vessel count and not a predictor of survival or recurrence in pancreatic carcinoma19). Also, Fujimoto et al reported that VEGF expression did not correlate with various clinicopathological parameters, including the depth of tumor invasion, stage, lymph node metastasis or hepatic metastasis20). A discrepancy still exists on the prognostic value of VEGF in pancreatic carcinoma, according to our and other reports. Further studies are warranted to determine the prognostic relevance of VEGF.
Tumor formation and growth are characterized by uncontrolled cellular proliferation. This is usually the result of multiple genetic and epigenetic insults to the cell, particularly involving proto-oncogenes and tumor suppressor genes. The p53 tumor suppressor gene is believed to play a pivotal role in preventing the uncontrolled cellular growth characteristic of a tumor26–29). p53 gene is mutated or deleted in about 50% of spontaneously arising tumors26–29). Since the cellular half-life of wild type p53 protein is very short and only a very small amount is present in a given cell, normal mucosa is not stained immunohistochemically using monoclonal antibody against this protein. Conversely, the mutated p53 protein accumulates in the nucleus through binding to other oncogenic proteins or by prolonging its half-life28). p53 protein overexpression detected by immunohistochemistry is a prognostic factor in many human neoplasms, such as gastric, colon and pancreatic carcinomas18,38,39).
In our study, the positive expression of p53 was detected in 18 of 30 (60.0%) pancreatic carcinoma tissues analyzed. However, no significant correlation was found between p53 expression and various clinicopathological parameters. There are several possible explanations for this discrepancy. This may in part be due to the relatively small sample size. The protein expression detected by immunohistochemistry is not always in accordance with gene status. Also, the steps of tumor growth and progression are not dependent on mutation of tumor suppressor gene alone and are regulated by many biological processes, including apoptosis, angiogenesis and invasion.
The angiogenic switch is regulated by changes in the relative balance between inducers and inhibitors of endothelial cell proliferation and migration6,7). The switch can be activated by increasing the levels of inducers, such as VEGF and/or by reducing the concentration of inhibitors, such as thrombospondin-16,7). Recently, the status of p53 has been implicated in the regulation of angiogenesis. The presence of wild-type p53 has been associated with the inhibition of angiogenesis, both by increasing expression of thrombospondin-1 and decreasing that of VEGF30–34).
Previously, Fujioka et al demonstrated that p53 expression not only was closely related to VEGF expression but also positively correlated with intratumoral microvessel density in pancreatic carcinoma18). Also, our study confirmed a significant correlation between mutant p53 protein and VEGF expression. Thus, p53 mutation during pancreatic tumorigenesis deregulates both arms of the balance, providing a potent stimulus for angiogenesis and tumor progression, although no significant correlation was found between p53 expression and various clinicopathological parameters.
In summary, VEGF, a key factor for the induction of tumor-associated angiogenesis, may be involved in tumor characteristics, including tumor invasion and metastasis. And p53 mutation may be implicated in regulation of angiogenesis through a VEGF up-regulation.