A common mutation in cholesteryl ester transfer protein gene and plasma HDL cholesterol level before and after hormone replacement therapy in Korean postmenopausal women
Article information
Abstract
Background
Plasma cholesteryl ester transfer protein (CETP) functions to transfer cholesteryl ester from HDL to trigiyceride-rich lipoproteins and regulates plasma HDL cholesterol level. A common mutation, the exon 15 A to G substitution at codon 442 (D442G) results in reduced plasma CETP activity and increased plasma HDL cholesterol level. Meanwhile, hormone replacement therapy (HRT) in postmenopausal women increases plasma HDL cholesterol level.
Methods
We investigated the frequency of D442G mutation and its effect on plasma HDL cholesterol level in Korean women. We also examined if the mutation has any effect on an increase in plasma HDL cholesterol level during HRT.
Results
Two hundred and twenty eight women aged over 40 years were recruited in this study. Of 228 women, 22 (9.6%) were identified as having the D442G mutation; 21 heterozygotes and 1 homozygote. The subjects with the mutation had higher plasma HDL cholesterol levels than those without the mutation (61.6±17.3 vs. 55.1±14.0 mg/dL, p<0.05). After 12 month HRT, HDL cholesterol increased by 6.4% (3.6±13.2 mg/dL, p<0.05) and D442G mutation did not have any significant effect on the change of plasma HDL cholesterol level.
Conclusion
D442G mutation is common in Korean postmenopausal women and it is associated with increased plasma HDL cholesterol level. HRT for postmenopausal women increased plasma HDL cholesterol level in similar amounts regardless of the presence or absence of D442G mutation.
INTRODUCTION
Epidemiologic studies have shown that high density lipoprotein (HDL) cholesterol is a strong negative risk factor for atherosclerotic vascular disease1, 2). The metabolism of HDL is complex and regulated by its apolipoprotein synthesis and catabolism, lipoprotein lipase, hepatic lipase activities, plasma lecithin cholesteryl acyltransferase activity and plasma cholesteryl ester transfer protein (CETP).
CETP functions to transfer cholesteryl ester from HDL to triglyceride-rich lipoproteins and regulates plasma HDL cholesterol level. The human CETP gene consists of 16 exons encompassing 25,000 bases on chromosome 16q133). A number of mutations in the CETP gene have been described since the first report in 19904). These mutations have been found not only in the Japanese population5) but also in the Caucasian population6, 7). The exon 15 A to G substitution at codon 442, base pair 1506 (D442G), is one of common mutations in the general Japanese population8, 9). D442G mutation results in plasma CETP deficiency and increased plasma HDL cholesterol level.
Plasma HDL cholesterol level in women is affected by hormonal status. Hormone replacement therapy (HRT) apparently increases plasma HDL cholesterol level10), even though the most recent studies did not resolve the question of whether HRT will prevent coronary heart disease.
In this study, we investigated the frequency of D442G mutation and its effect on plasma HDL cholesterol level in Korean postmenopausal women. We also studied if the mutation has any effect on an increase in plasma HDL cholesterol level during HRT.
METHODS
Subjects
Two hundred and twenty eight women were recruited in the climacteric clinic. They were naturally or surgically menopausal; if naturally menopausal, at least 12 months or with follicular stimulating hormone (FHS) level over 40 IU/L; if surgically menopausal, with FSH over 40 IU/L. Women who used estrogen, progestin or any medications that would influence lipid metabolism were excluded. Women with hypertriglyceridemia (400 mg/dL) were also excluded.
After baseline evaluation, they received one of the following treatments in 1-month cycles: (1) CEE (conjugated equine estrogen) 0.625 mg/d for 1 month (2) CEE 0.625 mg/d for 25 days plus medroxyprogesterone acetate 5 mg/d for the last 10 days followed by 5 days off (3) CEE 0.625 mg/d plus medroxyprogesterone acetate 5 mg/d for 1 month (4) Estradiol valerate 2 mg/d for 21 days plus cyproterone acetate 1 mg/d for the last 10 days followed by 7days off.
DNA analysis
Genomic DNA was prepared from peripheral buffy coats using a commercial kit (Takara Dr. GenTLE, Shiga, Japan). Polymerase chain reaction (PCR) was performed using the oligonucleotide primers, as previously described by Akita9). Fifty microliters of PCR reaction mixture included 1 mg genomic DNA, 100 pmol of each primer, 2 U Taq polymerase (SR products, UK), 200 mmol/L each deoxynucloside triphosphate, 25 mmol/L Tris-HCl, pH 8.0, 50 mmol/L KCl, 1.5 mmol/L MgCl2. Ten microliters of amplified products (30 cycles at 95°C for 40 sec, 62°C for 30 sec and 72°C for 30 sec) were generated by a thermocycler (Perkin Elmer, USA) and were digested with 10 U MspI (Takara Shuzo Co., Ltd., Shiga, Japan) at 37°C for 3 h. The DNA fragments were analyzed by electrophoresis on 3% NuSieve agarose gel (FMC, USA) stained by ethidium bromide and visualized using a standard ultraviolet transilluminator.
Measurement of serum lipids
Blood samples were obtained after a 12-h overnight fast. Serum cholesterol and triglyceride were determined enzymatically using a Hitachi 717 automatic analyzer. HDL cholesterol was measured directly by a method based on selective affinity of detergent to different lipoproteins (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan). Low-density lipoprotein cholesterol was calculated by the formula of Friedwald, et al11).
Statistical analysis
Statistical analyses were performed with the SPSS 7.5 for Windows. Values were expressed as mean±S.D. Differences in basal lipid levels and lipid changes after HRT between two groups according to D442G mutation status were assessed using t-test and differences in lipid levels between pre- and post-treatment were assessed using paired t-test. A p value less than 0.05 was considered statistically significant.
RESULTS
Patients’ average age was 52.1±3.6 years and average BMI (body mass index) was 23.7±2.7 kg/m2.
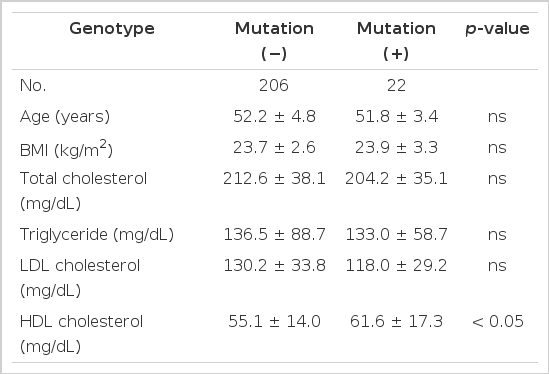
Of 228 women, 22 (9.6%) were identified as having the D442G mutation; 21 heterozygotes and 1 homozygote. The group with the mutation was not different in age and BMI from those without it. The subjects with the mutation had higher plasma HDL cholesterol levels than those without the mutation (61.6±17.3 vs. 55.1±14.0 mg/dL, p<0.05), but total cholesterol, triglyceride and LDL cholesterol levels did not differ between the two groups (Table 1).
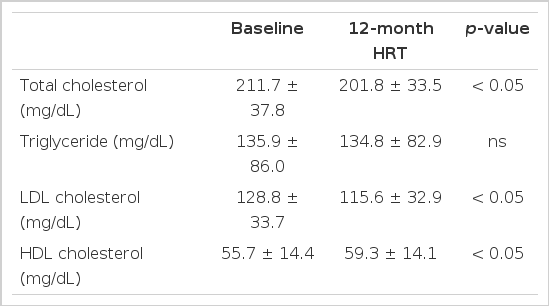
After 12-month HRT, plasma HDL cholesterol levels on the whole increased by 6.4% (3.6±13.2 mg/dL) and total cholesterol and LDL cholesterol levels decreased by 4.6% (9.9±34.0 mg/dL) and 10.2% (13.2±37.5 mg/dL), respectively. However, triglyceride level remained unchanged (Table 2).
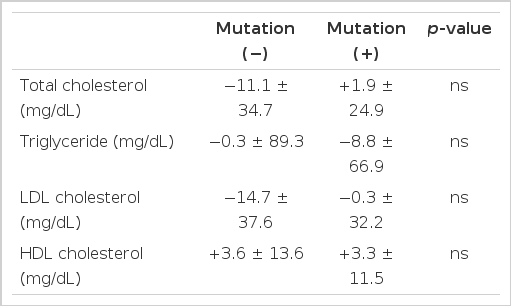
The mutation did not have any significant effect on the change in plasma HDL cholesterol level as well as total cholesterol, LDL cholesterol and triglyceride levels during HRT (Table 3).
DISCUSSION
The reverse transport of cholesterol from the periphery back to the liver is mediated by HDL. Cholesterol esters in HDL may undergo three different fates; removal by LDL receptors in the liver, tethering at the liver cell with subsequent selective uptake of HDL cholesterol and transferring cholesterol ester to triglyceride-rich lipoproteins as a result of the CETP activity. In humans, CETP pathway is relatively important. An inverse correlation between plasma CETP activity and plasma HDL cholesterol level has been well verified in normolipidemic subjects.
A number of mutations in the CETP gene have been identified, mostly in the Japanese and their descendants. The D442G mutation is very common in the Japanese general population (4.6–7%)8, 9), but it has not been found in Caucasian populations12). In our study, the D442G mutant was observed in 22 out of 228 Korean women (9.6%), which is similar to a prior report in the Korean population, including males13). One study reported its frequency (2.1%) in the Chinese14). To our knowledge, the D442G mutation has been found exclusively in Asians and their descendants.
Many mutations in CETP gene, including D442G mutation, result in reduced CETP activity, which consequently increases plasma HDL cholesterol level. This study showed that D442G mutation is associated with increased plasma HDL cholesterol level by 12% in Korean women. Initially, the D442G mutation was reported to cause as high as a three-fold increase in plasma HDL cholesterol level15). However, it is now evident that the D442G mutation typically results in moderate increase in plasma HDL cholesterol level in the Korean as well as the Japanese general population8, 9, 13).
Early reports of the CETP mutations suggested that they might be beneficial in that the resulting increased plasma HDL cholesterol level was associated with reduced cardiovascular risk. However, several recent reports16–18) suggest that individuals with CETP deficiency and high plasma HDL cholesterol level are associated with coronary artery disease. These studies challenged an early assumption that CETP deficiency, such as D442G mutation, might be beneficial, but the physiologic roles of CETP in atherogenesis are still not conclusive. It appears that the underlying mechanism involved in increasing HDL cholesterol may be important in determining whether the increase is protective or harmful.
The established effects of estrogen on blood lipids are an elevation in plasma HDL cholesterol level and a decrease in both total cholesterol and LDL cholesterol levels. Similarly, the results of this study showed that HRT was associated with an increase in plasma HDL cholesterol level. There are recent interesting reports that the effect of HRT on plasma HDL cholesterol level may override the effect of CETP genotype18, 19). There is also a report that CETP is not affected by HRT20). In this one year-follow-up study, HRT increased plasma HDL cholesterol level at similar amounts in women both with or without D442G mutation. Therefore, so far we cannot conclude on the interaction between CETP genotype and HRT on plasma HDL cholesterol levels.
In conclusion, D442G mutation is common in Korean women and it is associated with increased plasma HDL cholesterol level. The effect of HRT on plasma HDL cholesterol level was similar in women, both with or without D442G mutation.