Topotecan-Based Combination Chemotherapy in Patients with Transformed Chronic Myelogenous Leukemia and Advanced Myelodysplastic Syndrome
Article information
Abstract
Background
Patients with transformed chronic myelogenous leukemia(CML) and advanced myelodysplastic syndrome(MDS) have poor prognosis. The aim of this study is to evaluate the feasibility of second chronic phase induction in accelerated phase(CML-AP) or blastic crisis of CML(CML-BC) and remission induction in advanced MDS by combining topoisomerase I inhibitor(topotecan) with topoisomerase II inhibitor(mitoxantrone).
Methods
Twenty-four evaluable patients were entered on this study with a median age of 34 years. Eighteen patients with transformed CML(7 CML-AP, 11 CML-BC) and 6 patients with advanced MDS were treated. Topotecan was administered as 1.5 mg/m2/day by continuous infusion over 24 hours daily for 5 days every 4 to 8 weeks until remission. To enhance the tumoricidal effects, mitoxantrone(12 mg/m2/day, Days 1–3) was added.
Results
Eight patients(33%) achieved a complete remission(CR). Four of 7 patients with CML-AP(57%), 2 of 4 patients with CML-lymphoid blastic crisis (-LBC) (50%) and 2 of 6 patients with advanced MDS(33%) had CR lasting more than 45 days(45 to 400 days). There was no CR in the patients with CML-myeloid blastic crisis(-MBC). The dose level of 1.5 mg/m2/day(7.5 mg/m2/course) of topotecan was well tolerated in all patients. Mucositis occurred in 69% of patients (severe in 5%) and diarrhea in 67%(severe in 8%). In addition, there were no new or unexpected toxicities in the patients who were treated at this dose(7.5 mg/m2/course). In patients who recovered their neutrophil count, the absolute neutrophil count(ANC) remained below 500/μL for a period of 13 to 58 days(median 21 days) and the time to ANC recovery was associated with pretreatment severity of bone marrow fibrosis(mainly CML patients). Likewise, in the patients who recovered unsupported platelets, the platelets remained below 20,000/μL for a period of 0 to 37 days (median 19 days).
Conclusion
The combination of topotecan-mitoxantrone has shown modest activity in CML-AP, CML-LBC and advanced MDS with acceptable toxicities.
INTRODUCTION
Topotecan is a semisynthetic analog of the plant alkaloid camptothecin. The addition of a basic dimethylaminomethyl group on the A-ring of camptothecin gives topotecan enhanced water solubility compared with the parent compound. This drug is an inhibitor of topoisomerase I, an enzyme important in relieving the topologic strain of supercoiled DNA that occurs, for instance, during replication1). Topoisomerase I inhibitors deplete the cellular levels of active enzyme2), and they are believed to result in a compensatory increase in topoisomerase II activity3). Since reduced expression of topoisomerase II is an important cause of resistance to drugs that interact with that enzyme, it has been predicted that combining treatment with topotecan will sensitize cancer cells to drugs, such as anthracyclines and epipodophyllotoxins, that act by stabilizing complexes between topoisomerase II and DNA3,4). Therefore, topotecan combination with topoisomerase II inhibitors (e.g. anthracyclines and etoposide) may produce a DNA “double hit”, and using topotecan before topoisomerase II inhibitors could increase topoisomerase II enzyme levels and enhance drug efficacy, although in vitro studies have been inconclusive5). Topoisomerase II makes double strand breaks allowing the separation of daughter strands following DNA replication. Several important classes of anti-cancer drugs, including anthracyclines (such as doxorubicin or mitoxantrone) and epipodophyllotoxins (such as etoposide; VP-16), act by stabilizing the cleavable complexes between the ends of these DNA breaks and the topoisomerase II proteins, thereby preventing the religation of the breaks6). In addition, topotecan could be used in combination with cytarabine or with hypomethylating agents, because of the known clinical efficacy of these agents7,8), and in vitro synergism of such combinations9).
Phase I studies of topotecan in patients with solid tumors demonstrated that myelosuppression was the dose-limiting toxicity, and extramedullary toxicity was minimal10–13). Subsequent phase I studies of topotecan as a 5-day continuous infusion in patients with acute leukemia showed that significant dose escalation above that tolerated by patients with solid tumors was possible14,15). Since preliminary phase I studies of topotecan as a single agent demonstrated significant antileukemic activity, further evaluation of topotecan in combination with other antileukemic agents is warranted.
Clinical evaluations of topotecan-based combination chemotherapy regimens published to date have included topotecan combined with alkylating agents, platinum compounds, antimicrotubule agents, antimetabolites, anthracyclines, and epipodophyllotoxin, topoisomerase II-targeting agents. These agents have different mechanisms of action16) and demonstrated varying degrees of synergy when combined with topotecan in preclinical studies17. To the extent that toxicities have been additive or overlapping, dose adjustments have been necessary in these combination trials.
For patients with transformed chronic myelogenous leukemia (-accelerated phase, CML-AP; -blastic crisis, CML-BC), prognosis with conventional chemotherapy is poor. Patients with unfavorable prognostic features in MDS also have a median survival time of 3 to 6 months. The most important prognostic factors in those patients are duration of first remission, and achievement of subsequent complete remission (CR) prior to bone marrow transplantation(BMT), if an HLA-matched donor is available. We conducted in a clinical trial for patients with CML-AP or -BC and advanced MDS, treated with 5-days infusion of topotecan, a camptothecin analogue, combined with topoisomerase II inhibitor.
We report our single institutional preliminary results of the first 24 patients.
MATERIALS AND METHODS
Study group
Patients with a diagnosis of CML-AP or -BC and advanced MDS, referred to our institution between March 1998 and December 1998, were entered in the study (Table 1). Entry criteria were as follows: (1) age older than 15 years; (2) diagnosis of CML-AP or -BC, advanced MDS (RAEB, RAEB-T), or CMML, and (3) good performance status (ECOG; Eastern Cooperative Oncology Group≤2), normal hepatic (bilirubin level≤1.5 mg/dL) and renal (creatinine≤1.5 mg/dL) function. Patients with CML-AP were required to have the following: an increase in blast cells (> 15%) or basophils (>20%) in the blood or bone marrow, thrombocytopenia (<100,000/μL), severe anemia(hemoglobin[Hb] < 7 g/dL); documented extramedullary leukemia, or development of clonal evolution (new chromosomal changes in addition to the Ph1 chromosome). The presence of > 30% blasts in the bone marrow or peripheral blood was defined as blastic crisis(BC). BC was divided into two subgroups, lymphoid (CML-LBC) or myeloid blastic crisis(CML-MBC), based on morphologic finding and immunophenotyping. Patients with CMML were required to have an absolute monocyte count greater than 1 × 103/μL, in addtion to ≥8% monocytes in the blood or marrow18).
The pretreatment work-up included a history and physical examination, complete blood cell counts with differential, serum chemistries and bone marrow studies, including cytogenetic analysis with at least 25 metaphases, when available. During remission induction, blood counts were repeated daily and serum chemistries one to two times weekly. Marrow aspirations were performed at admission before the next scheduled chemotherapy. Other studies were performed as indicated by the patient’s clinical status.
Therapeutic protocol
Patients received topotecan 1.5 mg/m2 by continuous intravenous infusion daily for 5 days (7.5 mg/m2/course). To enhance the tumoricidal effects by combining topotecan, mitoxantrone(12 mg/m2/day, Day 1–3) was added. The courses were repeated every 4 to 8 weeks based on the following: (1) evidence of persistent disease on peripheral blood finding or on marrow studies, and (2) resolution of side effects. Induction therapy was to be discontinued if grade 3 to 4 toxicity occurred in the first course without CR, or if a response was not seen after a second course. In patients whose disease responded, two additional courses were continued every 4 to 6 weeks as maintenance course. The dose was reduced by 25% for grade 2 toxicity and by 50% for grade 3 to 4 toxicity.
Response and toxicity criteria
Criteria for CR was identical to those used in acute leukemia14). CR was defined as normalization of the marrow and peripheral blood with ≤5% marrow blasts, a granulocyte count greater than 103/μL, a platelet count greater than 100 × 103/μL and normal differential, with the response lasting for at least 4 weeks. Response in CMML also required a reduction of the absolute monocyte count to less than 1.0 × 103/μL and to less than 4% in the blood and marrow.
Toxicity was graded according to the criteria of the National Cancer Institute19). Remission duration was calculated from time of response achievement to documentation of disease recurrence.
RESULTS
Study group
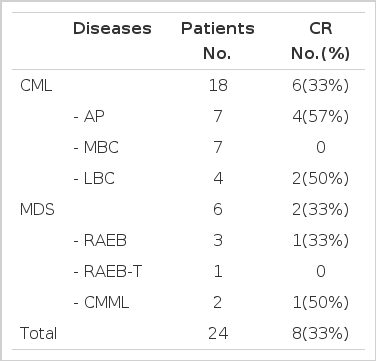
24 patients were treated, including 18 with CML(-AP, n=7; -LBC, n=4, -MBC, n=7) and 6 with advanced MDS (CMML, n=2; RAEB, n=3; RAEB-T, n=1). Patient characteristics are listed in Table 1. The median age was 34 years (range, 19 to 70); 10 patients were women.
Response to therapy and outcome
Eight out of 24 evaluable patients achieved CR (33%) (Table 2). No patients died early within 2 weeks. CR was observed in six patients (33%) out of 18 CML, including four of 7 CML-AP (57%), 2 of 4 CML-LBC (50%) and none of 7 CML-MBC patients. In advanced MDS, 2 of 6 patients achieved CR (33%). The number of courses given to patients on study was one to three (total 39 courses). The median follow-up duration was 13 months (range 7 to 16.5 months) and the CR duration was more than 45 days (45 to 400 days, median 120 days). Four of 6 CML CR patients who had HLA-matched donor received stem cell transplantation (3 HLA-matched unrelated BMT, 1 HLA-matched sibling BMD. Additionally, one patient with CML-AP did not achieve CR with topotecan and mitoxantrone, but achieved CR by following interferon treatment and received unrelated BMT.
Side effects
Since many patients had marrow compromise at the start of therapy, we investigated the hematologic profile recovery among responders. The median time to recovery of granulocyte to greater than 0.5 × 103/μL was 21 days(range, 13 to 58). The median time to recovery of platelet to greater than 20 × 103/μL was 19 days(range, 0 to 37). The hematopoietic recovery time was associated with severity of bone marrow fibrosis before treatment in CML patients. In the presence of myelofibrosis in 7 CML patients(1 CML-AP, 3 CML-MBC, 2 CML-LBC), the median time to recovery of ANC more than 0.5 × 103/μL was 35 days(16 – 58 days) and platelet more than 20 × 103/μL was 31 days(27–37 days). No life-threatening bleeding was observed in any patient. Death attributed to myelosuppression-associated complication did not occur.
Nonhematologic toxicities were mucositis 69%, which was severe(grade 3 to 4) in 5%, and diarrhea(67%; severe 8%). Nausea and vomiting was reported in 37%, but was severe in only 3%(Table 3). No other serious side effects occurred.
DISCUSSION
The results of this study demonstrate modest activity of topotecan in a 5-day schedule topoisomerase II inhibitor in patients with CML (-AP, -LBC), and advanced MDS(57% in CML-AP, 50% in CML-LBC, and 33% in advanced MDS). Of all evaluable patients, topotecan- combining chemotherapy (combined with mitoxantrone) produced a 33% response rate. In addition to the 8 patients who had CR, one patient with CML-AP achieved no definitive CR but interferon maintenance therapy allowed CML-CP transformation until HLA-matched unrelated BMT was performed. No CRs were observed in CML-MBC. Topotecan was well tolerated and safely administered. In addition, there were no new or unexpected toxicities in the patients who were treated at a dose of 7.5 mg/m2/course. This is particularly significant because CR was observed among patients with advanced hematologic diseases, such as transformed CML and advanced MDS, and topotecan has a completely different mechanism of action from agents known to be active in myeloid malignancies (topoisomerase II reactive agents). Treatment of patients with the above diseases with conventional chemotherapies has rarely produced normalization of all blood counts or increased survival. Although BMT results in long term-leukemia-free survival in some patients, the most important prognositc factor influencing the outcome after BMT is whether CR can be achieved prior to BMT.
In this study, the efficacy of topotecan-combining treatment for patients with transformed CML, advanced MDS and CMML was evaluated. Topotecan combined with topoisomerase II inhibitor, mitoxantrone, had effectiveness for CML-AP or -LBC and advanced MDS. After achieving CR in transformed CML patients, 4 of 6 patients received BMT from matched unrelated donors and are now well.
CONCLUSION
Topotecan with topoisomerase II inhibitor has shown modest activity in CML-AP or -LBC and advanced MDS with acceptable toxicity. To encourage synergism on the bases of topoisomerase pharmacokinetics, future studies will investigate topotecan-mitoxantrone (topoisomerase I and II inhibitors) sequential and dose escalation treatment for relapsed or refractory acute leukemia and CML-MBC.