Digital ulcerations in amyopathic dermatomyositis
Article information
A 51-year-old woman was referred to the rheumatology department for persistent digital ulcers that showed no improvement despite treatment with glucocorticoids and azathioprine. Six months prior, the patient had presented with dyspnea and dermatitis at the pulmonology department, where she was diagnosed with interstitial lung disease (ILD) and completed treatment. She presented with ulcerations on the interphalangeal joints on the volar side of her fingers and violaceous erythematous, scaly, and crusted patches on the dorsal side of her both hands (Fig. 1). The patient exhibited no muscle weakness. A plain X-ray of the hands showed no bony abnormality. Follow-up chest radiographs and computed tomography scans showed no residual ILD lesions. A skin biopsy was performed on the right thumb and palm, revealing minimal inflammatory changes in the upper dermis and increased mucin deposition in the deep dermis (Fig. 2). Serum muscle enzymes, including creatine kinase, and inflammatory markers, such as C-reactive protein and erythrocyte sedimentation rate, were within normal ranges. Serologic tests were positive for anti-nuclear antibodies (a titer of 1:160) and anti-Ro52, but negative for other myositis-associated autoantibodies such as anti-Jo-1, anti-Ku and anti-PM-Scl. Among myositis-specific autoantibodies, only anti-melanoma differentiation-associated protein 5 (MDA5) was positive, whereas anti-Mi-2alpha, anti-Mi-2beta, anti-TIF1-gamma, anti-NXP2 and anti-SAE1 were negative. Peripheral angiography was conducted to evaluate arterial flow in both hands, revealing no definite obliteration of blood flows. Based on these test results, the patient was diagnosed with clinically amyopathic dermatomyositis (CADM) with anti-MDA5 positivity.
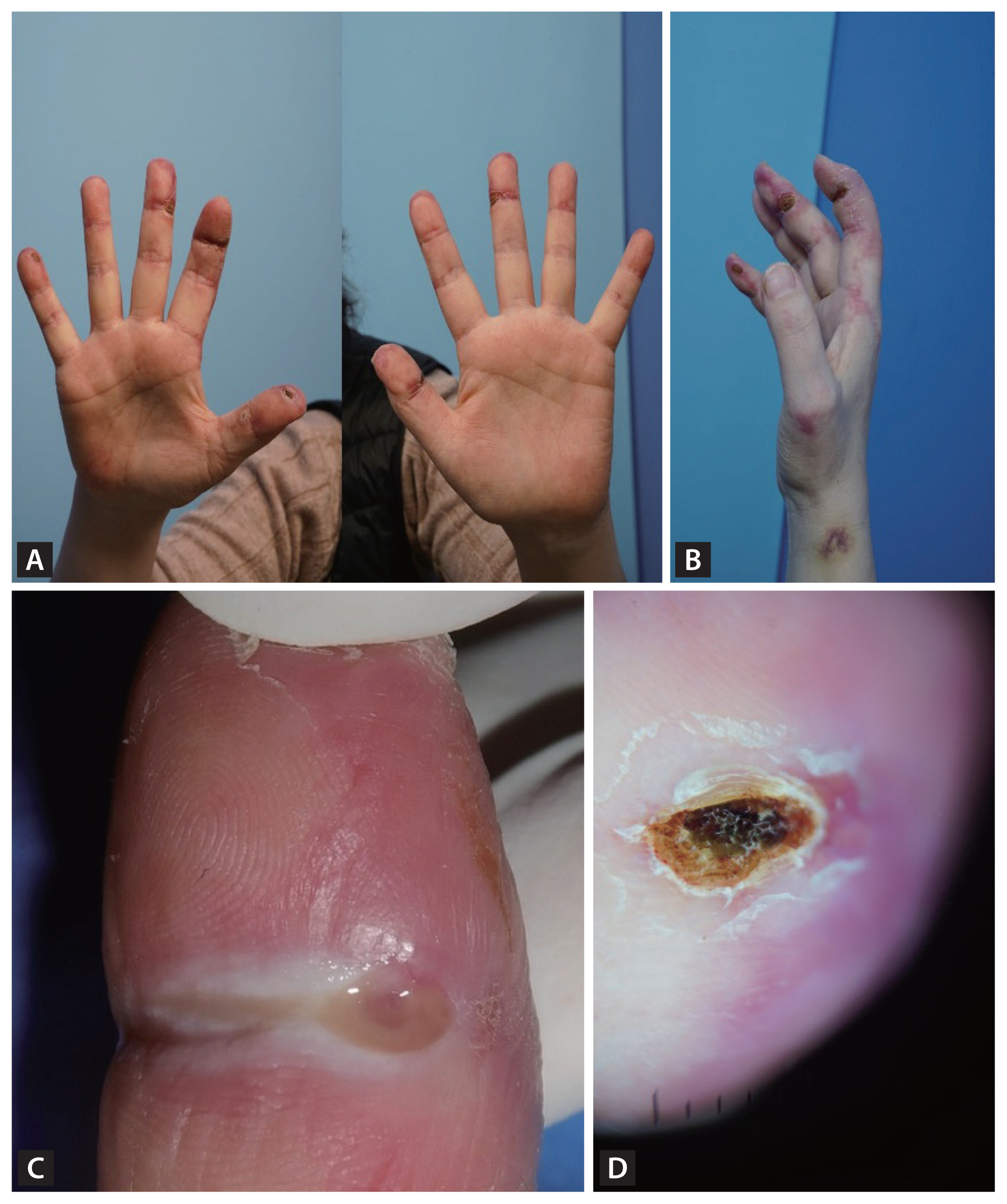
Distinctive digital ulcerations and patches in a patient with clinically amyopathic dermatomyositis (CADM). (A) Multiple ulcerations on the interphalangeal joints on the volar side of fingers. (B) Violaceous erythematous, scaly, and crusted patches on the dorsal side of the hand. (C) A close-up image of the ulcer on the right second digit revealing a well-demarcated, deep ulcer with erythematous borders and fibrinous exudate, characteristics of ischemic damages in CADM. (D) A dermoscopic image of the right thumb showing violaceous erythematous, scaly, and crusted patches with irregular vascular patterns and diffuse erythema.
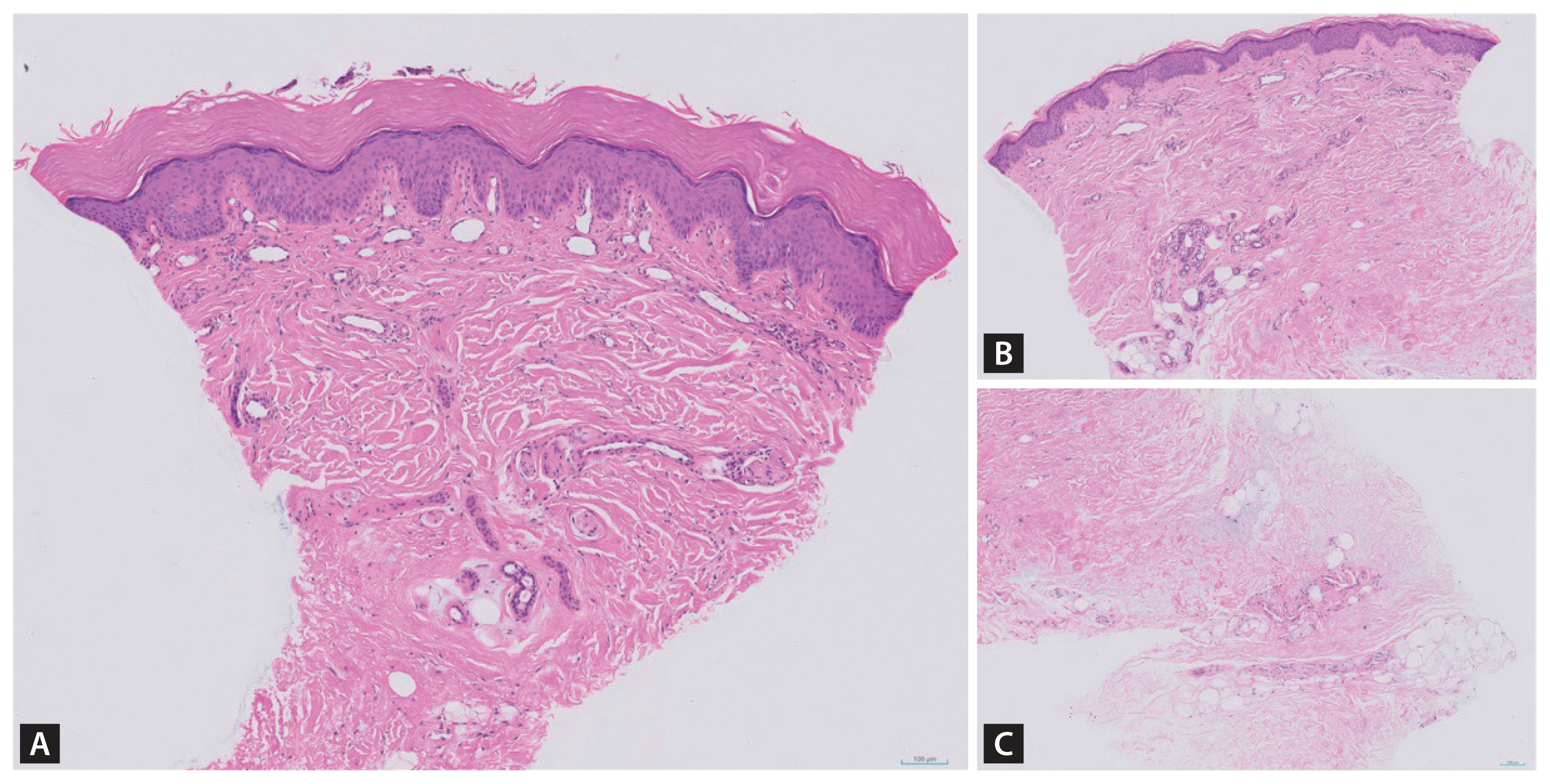
Histopathologic images from the skin biopsy specimens of right palm and thumb (hematoxylin and eosin, original magnification × 200). (A) Minimal perivascular lymphohistiocytic infiltration in the upper dermis of right palm. (B) Minimal perivascular lymphohistiocytic infiltration in the upper dermis of right thumb. (C) Focally increased dermal mucin in the deep dermis of right thumb.
Anti-MDA5 antibody-positive CADM is a subtype of dermatomyositis associated with progressive ILD and distinctive skin manifestations, including ulcerations [1]. In CADM, digital ulcers can affect both the digital pulp and areas with Gottron’s rash [2–4]. In the present case, previously treatment- resistant digital ulcers improved after six months of multiple immunomodulatory treatments including tacrolimus, systemic glucocorticoids, and hydroxychloroquine.
Notes
CRedit authorship contributions
Hye In Cho: writing - original draft; Ju Hee Han: visualization; Youngjae Park: writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None
