Clinical impacts of COVID-19 on severe exacerbation and mortality in interstitial lung disease: prognosis 30 days after infection
Article information
Abstract
Background/Aims
The impact of coronavirus disease 2019 (COVID-19) on severe exacerbation and mortality in interstitial lung disease (ILD) is unclear. In this study, we evaluate the risk of severe exacerbation and mortality in individuals with ILD following COVID-19.
Methods
Using the Korean National Health Insurance claim-based database, we compared the incidence and risk of severe exacerbation and mortality in individuals with ILD who survived at least one month after COVID-19 (COVID-19 cohort, n = 359) and 1:3 age, sex, and body mass index-matched individuals with ILD who did not have COVID-19 (controls, n = 1,077) between October 8, 2020, and August 30, 2021.
Results
During a mean follow-up of 7.4 months, the COVID-19 cohort had a higher risk of severe exacerbation compared to controls (aHR 2.26, 95% CI 1.38–3.69). During a mean follow-up of 19.6 months, the COVID-19 cohort had a higher risk of death (aHR 2.79, 95% CI 1.63–4.79) compared to controls. When considering COVID-19 severity, the severe COVID-19 group had a higher risk of severe exacerbation and death compared to controls, while the non-severe COVID-19 group did not show increased risk of severe exacerbation or death. In analyses based on ILD subtype, individuals with idiopathic pulmonary fibrosis in the COVID-19 cohort had the highest risk of severe exacerbation and death.
Conclusions
Previous severe COVID-19 was associated with worse clinical outcomes in individuals with ILD, especially in patients with idiopathic pulmonary fibrosis.
INTRODUCTION
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to a global pandemic of coronavirus disease 2019 (COVID-19), affecting millions of individuals worldwide [1,2]. Transmission rates have significantly decreased due to extensive global efforts, including vaccination campaigns and contact restrictions, ushering in a transition to an endemic phase [3]. However, the global health community continues to grapple with the health implications of SARS-CoV-2 infection—both in the acute phase and over the long term.
Interstitial lung disease (ILD) represents a diverse group of lung diseases characterized by progressive pulmonary fibrosis. Previous research has highlighted that individuals with pre-existing ILD, particularly those with idiopathic pulmonary fibrosis (IPF), face increased susceptibility to SARS-CoV-2 infection and are at greater risk of developing severe COVID-19 [4,5]. Furthermore, acute SARS-CoV-2 infection can trigger acute exacerbation of ILD [6,7], leading to unfavorable short-term outcomes in COVID-19 treatment for these patients [8-12].
While existing studies have primarily focused on COVID-19 susceptibility in ILD patients and clinical outcomes during the acute phase of the disease, clinicians must now confront management challenges posed by COVID-19 survivors with pre-existing ILD. Unfortunately, there remains a dearth of information regarding the post-acute risks of exacerbations and mortality in ILD patients who have recovered from COVID-19. Therefore, the goal of this study was to evaluate the risks of acute exacerbation and mortality in patients with ILD 30 days after COVID-19 using a nationwide cohort dataset in Korea.
METHODS
Data source
This retrospective cohort study utilized the Korean National Health Insurance claims database administered by the National Health Insurance Service (NHIS). The NHIS is a government-managed universal insurance provider, extending coverage to approximately 97% of the Korean population, encompassing around 50 million individuals [13,14]. Additionally, we obtained mortality information from the NHIS database, which is supplied by Statistics Korea under the auspices of the Ministry of Strategy and Finance of Korea [13,14].
The Korean government encouraged COVID-19 testing by providing financial assistance for diagnosing COVID-19 in individuals displaying symptoms suggestive of viral infection. Moreover, the Korean government provided financial support for the treatment of individuals confirmed to have COVID-19 (NHIS-2022-1-623). The NHIS SARS-CoV-2 database encompasses medical data for all individuals who underwent testing for the SARS-CoV-2 virus (n = 3,112,780), as well as those who were diagnosed with COVID-19 at least once from October 8, 2020, to August 31, 2021. Individuals who were not diagnosed with COVID-19 were extracted from the NHIS database using a randomized stratified sampling based on age and sex.
The study protocol was approved by the Institutional Review Board of Hanyang University Hospital (No. 2024-01-043). The requirement for informed consent was waived because all patient records were anonymized before use.
Pre-existing ILD was defined using claims with ICD-10 diagnosis code J84.x before the diagnosis of COVID-19 between October 8, 2010, and August 30, 2021. ILD subtypes were categorized as IPF (J84.1), connective tissue disease (CTD)-related ILD (J84.x codes with CTD codes [M05, M06, M31.5, M32, M33, M34, M35.0, M35.1, M35.3, M36.0, or M35.9]), and others (hypersensitivity pneumonitis [J67] and other ILDs) [4].
Study exposure: COVID-19
Laboratory diagnosis of SARS-CoV-2 infection was defined as a positive result from real-time RT–PCR assay of nasal or pharyngeal swabs from individuals with a history of SARS-CoV-2 (U071) defined using the ICD-10 [4,15,16]. Severe COVID-19 was defined as documented use of oxygen therapy, intensive care unit admission, mechanical ventilation treatment, or extracorporeal membrane oxygenation during hospitalization due to COVID-19 [4].
Study outcomes: severe exacerbation and mortality
The primary outcomes were severe exacerbation and all-cause mortality. Severe exacerbation was defined as emergency room visits or hospitalizations related to ILD and concomitant presence of oral or intravenous corticosteroid use.
For analysis of severe exacerbation, individuals were followed until the date of severe exacerbation, death, or December 2021, whichever came first. Death-related information was available through January 2023. Thus, for the mortality analysis, we tracked individuals until the date of death or January 2023, whichever came first. We used a 30-day lag period to evaluate the impact of COVID-19 on ILD beyond the acute phase.
Covariates
Data for baseline characteristics of sex, age, body mass index (BMI), smoking status, alcohol consumption, income status, and comorbidities were collected. BMI was calculated as body weight divided by the square of height (kg/m2) and was classified into four groups, as recommended for East Asians: normal (18.5–22.9 kg/m2), underweight (< 18.5 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥ 25kg/m2) [17]. Smoking status (never-, ex-, or current smoker) was determined based on a self-reported questionnaire [18].
The bottom 30% of individuals in terms of income status and individuals supported by the medical aid program were defined as the low-income group [19]. Comorbidities were defined using one or more claims under ICD-10 diagnosis codes as major diagnoses within one year of the index date [20-27]: airway disease (COPD [J42, J43, or J44 except for J430] and asthma [J45, J46]), diabetes mellitus (DM, E10–14), hypertension (I10–15), angina pectoris (I20), myocardial infarction (I21, I22, or I25.2), cerebrovascular disease (G45–G46, I60–I69, or H34.0), heart failure (I43, I50, I09.9, I11.0, I25.5, I13.0, I13.2, I42.0, I42.5–I42.9, or P29.0), CTD (M05, M06, M31.5, M32, M33, M34, M35.0, M35.1, M35.3, M36.0, or M35.9), lung cancer (C34), and malignancy other than lung cancer (C00–C97 except for C34). Charlson comorbidity index (CCI) was calculated as recommended [28,29].
Statistical analysis
Descriptive statistics are presented as numbers (percentages) for categorical variables. We compared two groups using the χ2 test for categorical variables. The incidence rates of severe exacerbation and death were calculated by dividing the number of incident events by the total follow-up period (per 1,000 person-years). A cumulative incidence plot was used to compare the incidences of severe exacerbation and death, and a log-rank test was utilized to evaluate significant differences between the two groups.
We conducted 1:3 matching between the COVID-19 cohort and controls based on age, sex, and BMI. Standardized mean difference (SMD) was used to examine the balance of covariate distribution between the two groups, with SMD > 0.1 indicating imbalance [30]. We used Cox proportional hazards regression analyses to evaluate the risk of severe exacerbation or death in the COVID-19 cohort vs. controls. The multivariable model was adjusted for smoking status, income status, and CCI scores. We also conducted stratified analyses based on sex, age, BMI, low income, smoking status, and CCI score. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were carried out using SAS Enterprise Guide software version 7.1 (SAS Institute, Inc., Cary, NC, USA) and R software, version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study population
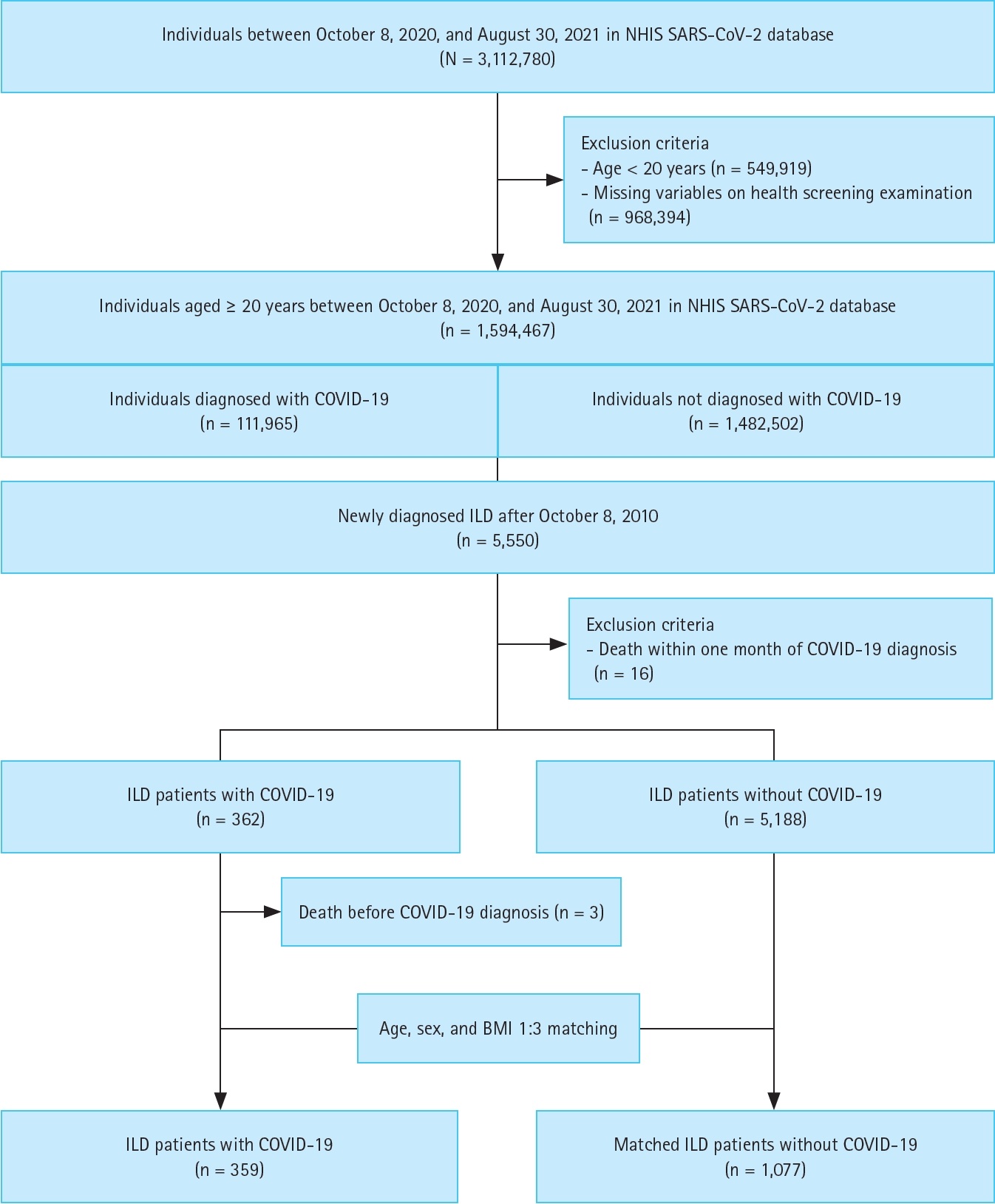
We used the NHIS SARS-CoV-2 database (n = 3,112,780) for our analysis. After excluding 549,919 individuals younger than 20 years and 968,394 individuals with missing demographic characteristics, a total of 1,594,467 individuals aged ≥ 20 years between October 8, 2020, and August 30, 2021, remained in the dataset (111,965 diagnosed with COVID-19 and 1,482,502 not diagnosed with COVID-19). Among them, we identified 5,550 individuals newly diagnosed with ILD after January 1, 2010. Next, we excluded 16 individuals who died within one month (lag period) of COVID-19 diagnosis. Consequently, 362 individuals with ILD were diagnosed with COVID-19 (COVID-19 cohort), and 5,188 individuals with ILD were not (control cohort). Within the COVID-19 cohort, three individuals who died before official diagnosis were further excluded, resulting in a final COVID-19 cohort of 359 individuals. To build a matched control group, we performed 1:3 matching based on age, sex, and BMI, creating a set of controls of 1,077 individuals without COVID-19 (Fig. 1).
Baseline characteristics
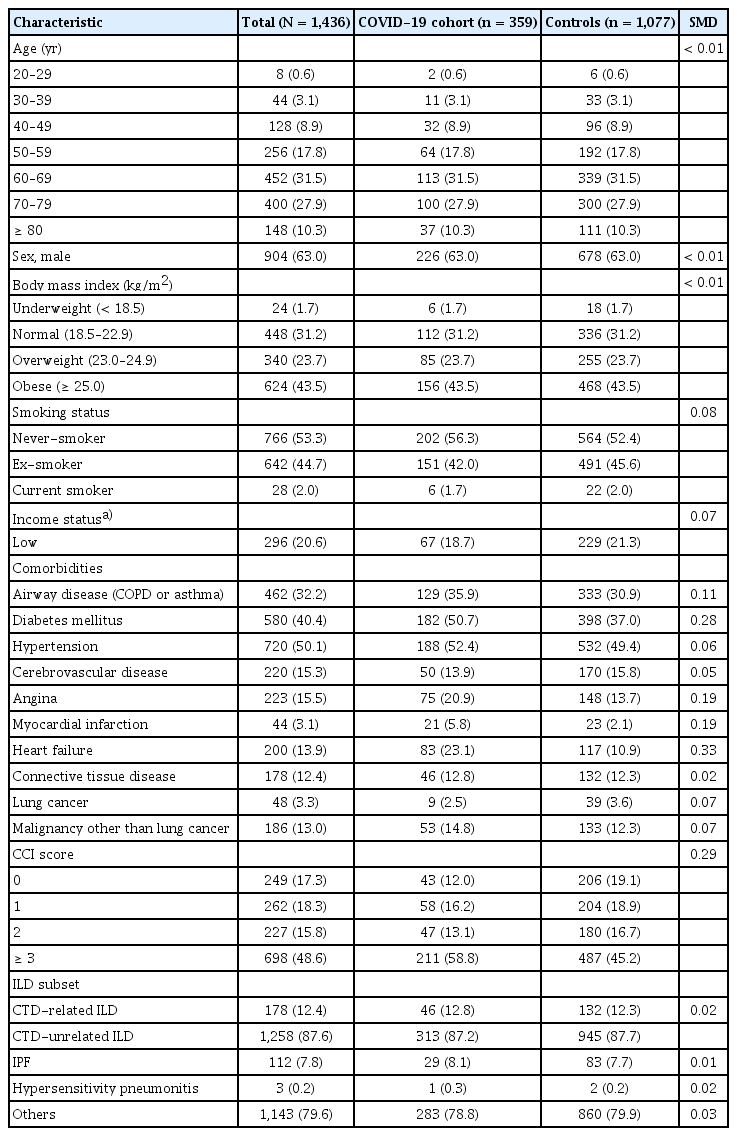
As shown in Table 1, there were no significant imbalances in age, sex, and BMI between the COVID-19 cohort and controls, with all SMDs less than 0.1. The COVID-19 cohort was more likely to have airway diseases, DM, angina, myocardial infarction, and heart failure compared to controls (all SMD > 0.1).
Impact of COVID-19 on severe exacerbation and mortality
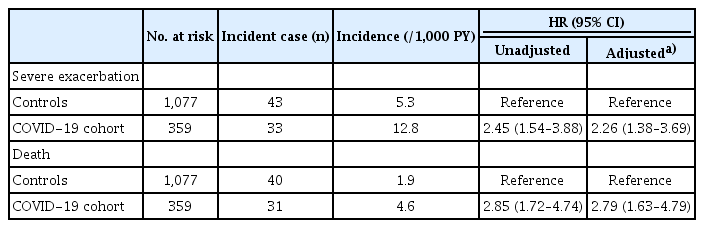
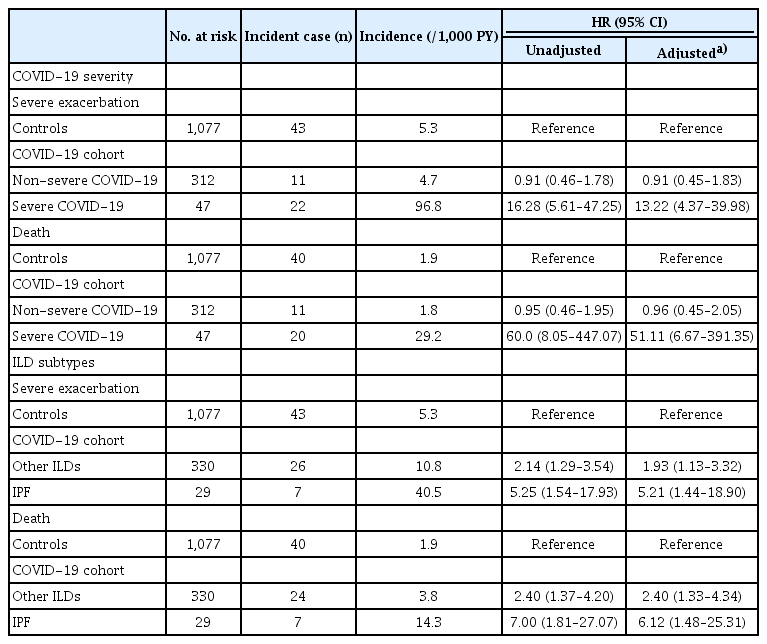
As shown in Table 2, during a mean follow-up of 7.4 (standard deviation [SD] 3.1) months including a 30-day lag period, the incidence rate of severe exacerbation was higher in the COVID-19 cohort than in controls (12.8/1,000 person-years vs. 5.3/1,000 person-years, p < 0.01). Even after adjusting for potential confounders, individuals in the COVID-19 cohort had a higher risk of severe exacerbation (adjusted hazard ratio [aHR] 2.26, 95% confidence interval [CI] 1.38–3.69) compared to controls.
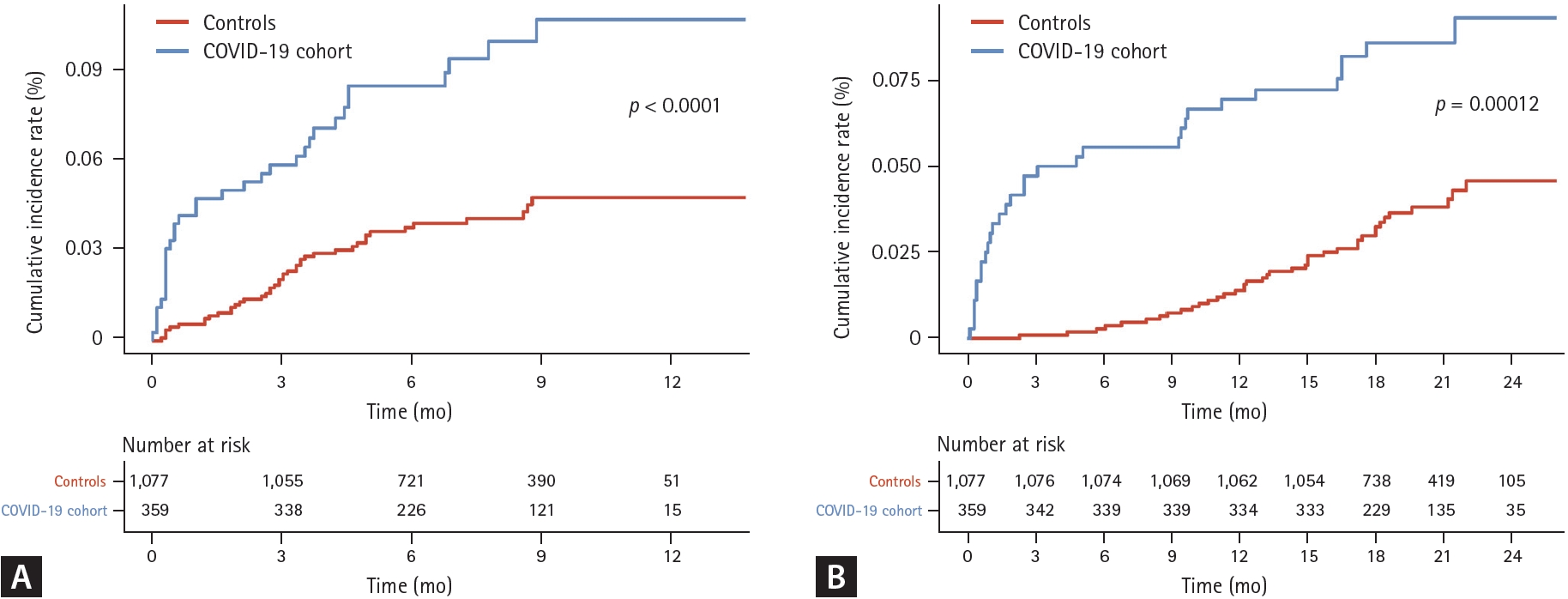
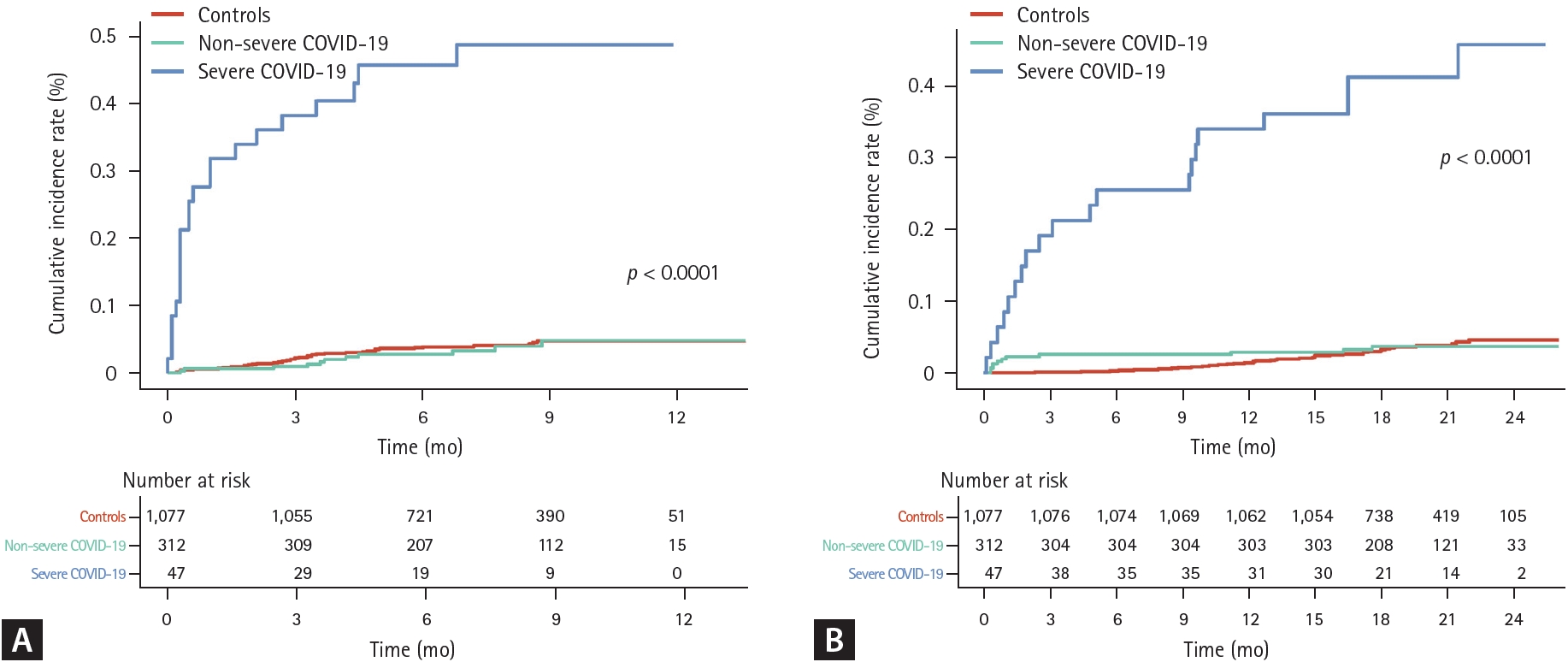
During a median follow-up of 19.6 (SD 3.9) months including a 30-day lag period, the incidence rate of death was higher in the COVID-19 cohort compared to controls (4.6/1,000 person-years vs. 1.9/1,000 person-years (p < 0.01). Individuals in the COVID-19 cohort had a higher risk of death (aHR 2.79, 95% CI 1.63–4.79) compared to controls (Table 2). The cumulative incidence plot for severe exacerbation and death showed similar results (Fig. 2, logrank p < 0.01 for both).
Impact of COVID-19 severity on severe exacerbation and mortality
The incidence rate of severe exacerbation was higher among individuals in the severe COVID-19 cohort compared to those in the non-severe COVID-19 group (96.8/1,000 person-years vs. 4.7/1,000 person-years, p < 0.01). While the severe COVID-19 cohort had a higher risk of severe exacerbation compared to controls (aHR 13.22, 95% CI 4.37–39.98), the non-severe COVID-19 cohort did not (aHR 0.91, 95% CI 0.45–1.83) (Table 3).
The incidence rates of death were 29.2 per 1,000 person-years for the severe COVID-19 cohort and 1.8 per 1,000 person-years for the non-severe COVID-19 cohort (p < 0.01). Compared to controls, the severe COVID-19 cohort had a higher risk of death (aHR 51.11, 95% CI 6.67–391.35), while the non-severe COVID-19 cohort did not (aHR 0.96, 95% CI 0.45–2.05) (Table 3). The cumulative incidence plot for severe exacerbation and death showed similar results (Fig. 3, log-rank p < 0.01 for both).
Impact of ILD subtype on risks of severe exacerbation and mortality
When analyzed by ILD subtype, the risk of severe exacerbation in the COVID-19 cohort compared to controls was 5.21 (95% CI 1.44–18.90)-fold and 1.93 (95% CI 1.13–3.32)-fold higher for IPF and non-IPF ILDs, respectively (Table 3). Individuals with IPF and those with non-IPF ILDs in the COVID-19 cohort had 6.12 (95% CI 1.48–25.31)-fold and 2.40 (95% CI 1.33–4.34)-fold increased risk for death compared to controls (Table 3). Similar results were shown in the cumulative incidence plots (Fig. 4, log-rank p < 0.01 for both).
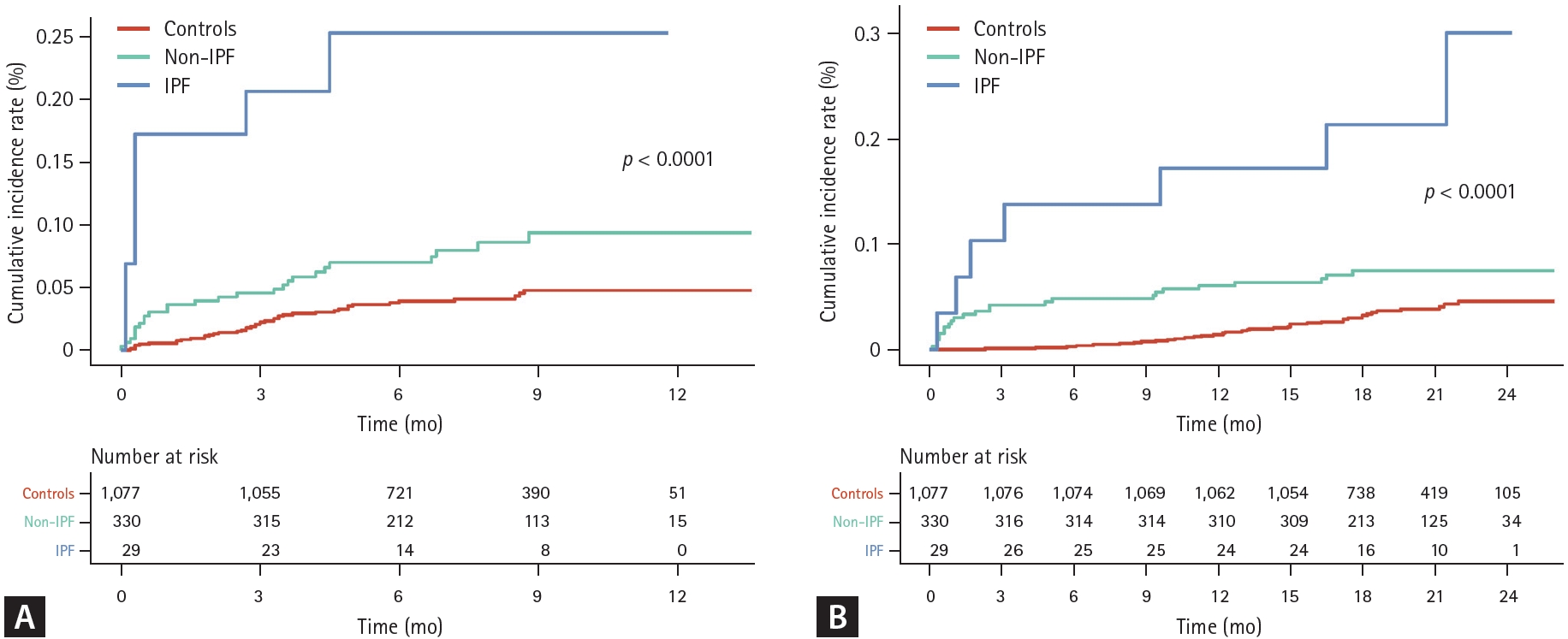
(A) Cumulative incidence of severe exacerbation according to ILD subtype. (B) Cumulative incidence of death according to ILD subtype. Zero on the X-axis indicates 30 days after COVID-19 diagnosis. ILD, interstitial lung disease; COVID-19, coronavirus disease 2019; IPF, idiopathic pulmonary fibrosis.
Subgroup analyses
Smoking status exhibited a significant interaction in the association of COVID-19 with death (all p for interaction < 0.05 for both) (Supplementary Table 1). The risk for mortality was especially higher in ever-smokers compared to their counterparts.
DISCUSSION
We provide a comprehensive assessment of how COVID-19 impacts the subsequent course of ILD in individuals with pre-existing ILD who survived the acute phase of COVID-19. Our findings revealed that individuals with ILD who survived COVID-19 faced more than a two-fold increased risk of severe exacerbation and mortality compared to those who did not have COVID-19. Notably, this heightened risk was specifically linked to severe COVID-19 but not to non-severe COVID-19. Both subtypes of IPF and other ILDs were associated with higher risk of severe exacerbation and death compared to controls. However, the risk was particularly pronounced in individuals with IPF.
During the COVID-19 pandemic era, previous studies primarily focused on COVID-19 susceptibility and clinical outcomes during the acute phase in individuals with ILD [4,6-12]. Overall, the evidence suggests that individuals with pre-existing ILDs may exhibit heightened susceptibility to COVID-19, and that underlying ILDs adversely affect the acute clinical course of the viral infection. For example, a noteworthy study demonstrated that patients with ILD had a four-fold higher incidence of severe COVID-19 and mortality compared to controls [4], underscoring the vulnerability of this patient population.
Suboptimal treatment outcomes naturally raise concerns about the consequences for ILD survivors following COVID-19. However, there is limited information regarding the impact of previous COVID-19 history on the course of ILD beyond the acute phase. Our study bridges this gap by providing clinically relevant data. We found that prior COVID-19 history significantly increases the likelihood of severe exacerbation and mortality in individuals with ILD. Remarkably, this association was specifically pronounced in patients who experienced severe COVID-19. No significant differences were noted in non-severe COVID-19 cases in severe exacerbations and mortality between the control and COVID-19 cohorts. This suggests that, in patients with ILD, we need to pay more attention to the management of those with severe COVID-19 beyond the acute phase.
When the underlying ILD subtypes were considered, COVID-19-related clinical outcomes were more pronounced in individuals with IPF compared to those with other ILDs, heightening the importance of the severity of COVID-19 as well as the underlying subtypes in the consequences of COVID-19 in ILD.
While the precise mechanisms underlying our observations require further investigation, we propose some hypotheses. The exclusive associations between severe COVID-19 and higher risks of severe exacerbation and mortality in ILDs suggest that patients experiencing non-severe COVID-19 may not experience clinically significant lung injury in the context of pre-existing ILD. In contrast, severe COVID-19, with its potential for damaging lung injury, could exacerbate existing pulmonary fibrosis and contribute to substantial pulmonary dysfunction [31,32]. The reasons for the highest risk of severe exacerbations and mortality in those with IPF remain elusive. However, during the acute phase of COVID-19, the clinical outcomes of COVID-19 were notably graver in patients with IPF compared to those with non-IPF ILDs [9]. Thus, more pronounced inflammation and lung injury during the acute phase of COVID-19 in IPF may have lasting consequences.
In subgroup analysis, the risk of mortality following COVID-19 recovery was greater among individuals with a history of smoking. Collectively, this suggests that a more pronounced acute phase of COVID-19-related inflammation and lung injury in this subpopulation may persists longer and adversely affects clinical outcomes beyond the acute phase.
In subgroup analysis according to age group, we observed a trend indicating a larger negative effect on survival in the COVID-19 group compared to controls, although this was not statistically significant. The lack of significant results may be due to the following factors. As shown in Table 1, most participants were aged 60–79 years, with only a small number aged 20–59. For subgroup analysis, we categorized participants into two groups: 20–69 years and ≥ 70 years. The small number of participants in the 20–59 age group and the limited number of deaths in this group may have impacted the analysis. We speculate that the results are less pronounced in younger age groups due to the higher prevalence of ILDs such as IPF, which are affected by COVID-19, in the ≥ 60 year age group. Larger datasets are needed for more detailed age-specific mortality-related analysis in the future.
Our study has two major strengths and provides novel insights in this field. First, we had an initial four-week lag period following COVID-19 diagnosis to include individuals who survived the acute phase of COVID-19. This allowed us to track the outcomes of ILD following COVID-19. Second, by stratifying COVID-19 cases based on severity, we found that only severe COVID-19 was associated with an elevated risk of severe exacerbation and mortality in individuals with ILD. Furthermore, by classifying ILD subtypes, we revealed that risks were particularly pronounced in those with IPF. Our results underscore the importance of close follow-up for individuals with ILD who have experienced severe COVID-19 and those with IPF following COVID-19. Given the poor prognosis associated with both acute and beyond acute COVID-19 outcomes in ILD, clinicians should prioritize efforts to prevent new COVID-19 cases in this vulnerable population [4,9,10,12].
Our study has several limitations. First, generalizing our findings to other countries or ethnicities may be challenging since our dataset was exclusively Korean. Second, the diagnosis of ILD and other comorbidities relied on ICD-10 codes, potentially leading to over- or underestimation of diagnoses. Additionally, we were unable to classify detailed subtypes of ILD in the “others” group. Third, since we lacked data on lung function, imaging, and medications for ILD (e.g., antifibrotic agents), the impact of these variables on the outcomes could not be evaluated. Future studies are needed in this regard. Fourth, there was no information about COVID-19 treatments (e.g., antiviral treatments) in the dataset. Thus, we were unable to analyze the impacts of COVID-19 treatments or treatment approaches on the outcomes.
We found that previous COVID-19 was associated with worse clinical outcomes, including severe exacerbation and death, in individuals with ILD who experienced severe COVID-19 or those with IPF. We suggest that clinicians remain vigilant in preventing COVID-19 in individuals with ILD and ensure close monitoring during follow-up, particularly after severe COVID-19 or in those with IPF.
KEY MESSAGE
1. In this study, we evaluated the clinical impact of COVID-19 on patients with ILD.
2. We found that ILD patients who survived COVID-19 were at higher risk of severe exacerbation and mortality, especially those with IPF or severe COVID-19.
3. Our findings suggest that COVID-19 survivors with ILD require careful management to mitigate these risks.
Notes
CRedit authorship contributions
Bo-Guen Kim: conceptualization, methodology, investigation, data curation, writing - original draft, writing - review & editing; Sun-Kyung Lee: conceptualization, methodology, data curation, formal analysis, visualization; Dong Won Park: conceptualization, methodology, writing - review & editing; Tai Sun Park: conceptualization, methodology, writing - review & editing; Ji-Yong Moon: conceptualization, methodology, writing - review & editing; Tae-Hyung Kim: conceptualization, methodology, writing - review & editing; Sang-Heon Kim: conceptualization, methodology, writing - review & editing; Ho Joo Yoon: conceptualization, methodology, writing - review & editing; Hyun Lee: conceptualization, methodology, resources, data curation, writing - original draft, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2025-00557268). This work was supported by the research fund of Hanyang University (HY-202500000001668).
Data availability
The raw data used for this study are available from the Korean National Health Insurance Service. Restrictions apply to the availability of these data, which were used under license for this study.