Impact of body mass index on clinical outcomes in intestinal Behçet’s disease
Article information
Abstract
Background/Aims
The impact of body mass index (BMI) on the clinical outcomes of intestinal Behçet’s disease (BD) remains unclear. This study assessed the association between BMI and disease progression.
Methods
A retrospective analysis of 760 patients with intestinal BD was conducted. Patients were classified by BMI as underweight (< 18.5 kg/m2), normal (18.5–22.9), overweight (23.0–24.9), or obese (≥ 25.0). The association between BMI and clinical outcomes—biologics, surgery, hospitalization, and emergency visits—was examined.
Results
Among 760 patients, 130 (17.1%) were underweight, 384 (50.5%) normal, 152 (20.0%) overweight, and 94 (12.4%) obese. Higher BMI linked to lower cumulative rates of biologics use (p trend = 0.002), surgery (p trend = 0.004), hospitalization (p trend = 0.004), and emergency visits (p trend = 0.008). Compared with the underweight group, the normal (HR 0.667, 95% CI 0.483–0.922, p = 0.014), overweight (HR 0.589, 95% CI 0.394–0.879, p = 0.010), and obese groups (HR 0.515, 95% CI 0.321–0.828, p = 0.006) had lower hospitalization risks. The overweight (HR 0.490, 95% CI 0.241–0.996, p = 0.049) and obese (HR 0.312, 95% CI 0.116–0.840, p = 0.021) groups were negatively associated with future biologics use. The normal (HR 0.705, 95% CI 0.480–1.036, p = 0.075) and obese (HR 0.510, 95% CI 0.272–0.953, p = 0.035) groups were negatively associated with future surgery in multivariable analysis.
Conclusions
Lower BMI was linked to poorer clinical outcomes in intestinal BD, emphasizing the need to optimize nutritional status.
INTRODUCTION
Behçet’s disease (BD) is a chronic, relapsing, multi-system inflammatory disorder characterized by recurrent oral and genital ulcers, ocular lesions, skin manifestations, arthritis, vasculitis, and gastrointestinal involvement. While BD is uncommon in Western countries, it is more prevalent in East Asia, particularly in Korea and Japan, as well as the Middle East and the Mediterranean. According to the Korean Association for the Study of Intestinal Diseases, intestinal BD is diagnosed in patients with systemic BD who exhibit typical endoscopic findings, such as one or a few oval-shaped ulcers with well-defined margins in the ileocecal region. If such ulcers are present and the patient has only oral ulcers, the condition is diagnosed as probable intestinal BD. In the absence of systemic BD, the diagnosis is suspected intestinal BD. If aphthous, shallow, atypical intestinal ulcers are observed in the ileocecal region along with systemic BD, the condition is still classified as probable intestinal BD. However, if only oral ulcers are present, the diagnosed remains suspected BD, and in the absence of systemic BD, it is categorized as nondiagnostic [1-4]. Approximately 5% to 10% of patients with BD have intestinal BD [5,6]. The significant clinical and pathological overlap between intestinal BD and inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), complicates differential diagnosis, requiring a deeper understanding of their similarities and differences to ensure accurate diagnosis and appropriate treatment [7,8].
Body mass index (BMI) is a widely used indicator of nutritional status and has been linked to systemic inflammation in various disorders. Previous studies have examined the relationship between BMI and the clinical outcomes of IBD, including UC and CD [9-11]. Malnutrition is a significant and common complication in IBD and is associated with decreased food intake, intestinal malabsorption, and increased energy requirements. The estimated prevalence of malnutrition in IBD varies significantly, ranging from 6.1% to 69.7%, depending on factors such as the malnutrition definitions, IBD type, clinical setting, and disease activity level [12-15].
Furthermore, IBD can severely affect a patient’s nutritional status, leading to adverse health outcomes. Malnutrition and sarcopenia have been directly associated with negative clinical outcomes, including increased hospitalization rates, reduced treatment response, and lower quality of life [13,16]. Due to these negative effects, research has focused on identifying and monitoring risk factors for malnutrition in IBD [12,17,18]. Given the similarities in the clinical progression of CD and intestinal BD, the prevalence of malnutrition and early initiation of nutritional support might be important in intestinal BD. However, few studies have investigated this relationship.
Recently, the prevalence of obesity in IBD patients has increased significantly, reflecting a broader trend in the general population. Studies estimate that 15–40% of IBD patients are now classified as obese, with BMI thresholds for obesity set at 30 kg/m2 or higher in Western populations [19]. However, the impact of obesity on IBD progression remains inconsistent, with studies reporting varying associations with disease severity, IBD-related surgery, hospitalization, and emergency department visits. Additionally, obesity may alter the efficacy of biological agents by increasing drug clearance, potentially leading to suboptimal trough concentration [11,20].
The coexistence of obesity and malnutrition within the same patient population presents a paradox that further complicates IBD management [19]. While extensive research has examined the relationship between obesity, malnutrition, and IBD, few studies have explored this relationship in intestinal BD. Given the overlapping clinical characteristics of intestinal BD and IBD, understanding how BMI influences clinical outcomes in intestinal BD is essential. This study aims to investigate the association between BMI and key clinical outcomes, including cumulative hospitalization rates, emergency room visits, surgery, and biologics use, in patients with intestinal BD.
METHODS
Patient population
This retrospective study included 760 patients diagnosed with intestinal BD who had available BMI records at the IBD Clinic of Severance Hospital in Seoul, South Korea, between 1997 and 2021. As this study was conducted retrospectively, informed consent was waived. The Ethics Committee of Severance Hospital approved this study (no. 4-2020-0686), which was conducted in accordance with the Declaration of Helsinki ethical guidelines.
Study design and definition of clinical outcomes
Medical records were collected, including age, sex, smoking status, history of appendectomy, family history, marital status, Disease Activity Index for Intestinal BD (DAIBD) scores at diagnosis, extraintestinal manifestations at diagnosis, presence of systemic BD, endoscopic ulcer characteristics, and laboratory findings. The primary clinical outcomes of interest were the cumulative rates of biologics use, intestinal BD-related surgery, intestinal BD-related hospitalization, and emergency room visits.
BMI was calculated as weight (kg) divided by height (m2), and patients were classified into four BMI groups based on Asia-Pacific standards [21]. The BMI categories were underweight (BMI < 18.5 kg/m2), normal (BMI 18.5–22.9 kg/m2), overweight (BMI 23.0–24.9 kg/m2), and obese (BMI ≥ 25.0 kg/m2). Each patient group was observed until a significant event occurred.
Statistical analysis
Continuous variables were expressed as means with standard deviations, while categorical variables were presented as frequencies and percentages. Baseline characteristics were compared across the four BMI groups using ANOVA or the chi-squared test. When significant differences were identified, Bonferroni correction was applied for multiple comparisons.
The occurrence rates of biologics use, surgery, hospitalization, and emergency room visits were analyzed using chisquare tests, and trends across BMI groups were examined. Kaplan–Meier curves and log-rank tests were used to assess differences in clinical outcomes during follow-up. A univariable Cox regression model was applied to evaluate the association between each variable and clinical outcomes. Variables with p < 0.05 in the univariable analysis were assessed for multicollinearity, ensuring that each had a Variance Inflation Factor below 10, confirming the absence of multicollinearity. A multivariable Cox proportional hazards model was then conducted to determine the independent associations between these factors and clinical outcomes. A p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
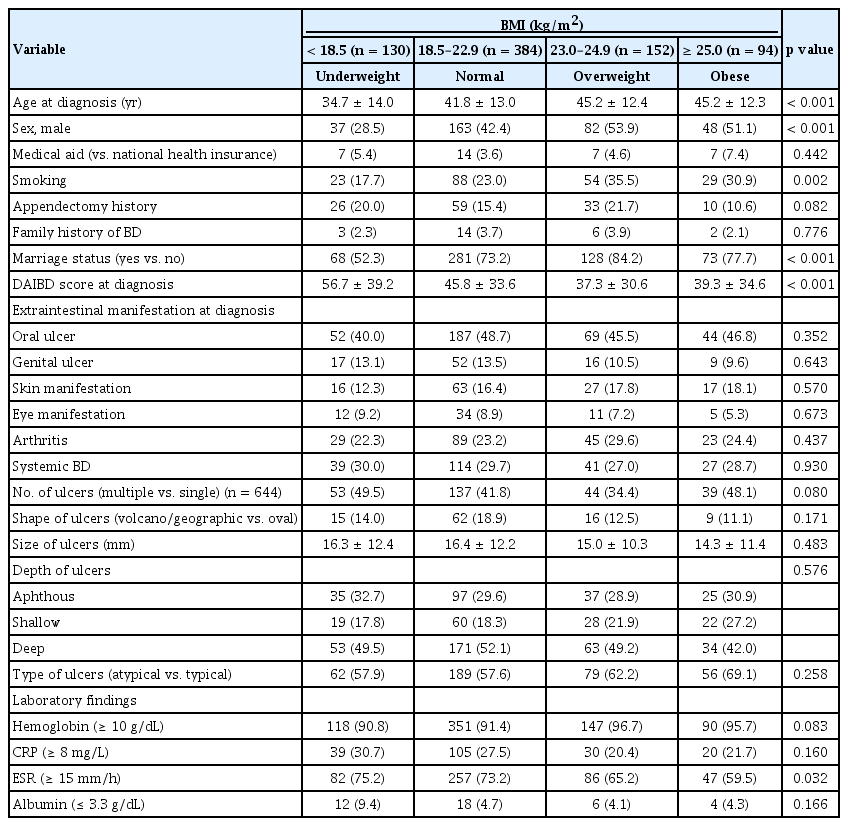
Our cohort comprised 43.3% males and had a mean age at diagnosis of 41.7 ± 13.4 years, a mean BMI of 21.6 ± 3.2 kg/m2, and a mean DAIBD score of 45.2 ± 34.7. Of the 760 patients, 130 (17.1%) were underweight, 384 (50.5%) were normal weight, 152 (20.0%) were overweight, and 94 (12.4%) were obese, based on their BMI classification (Table 1). The mean follow-up time for all patients was 12.5 ± 7.2 years, with a median follow-up of 12.1 years.
Baseline characteristics, including age, sex, smoking status, marital status, DAIBD score, and erythrocyte sedimentation rate (ESR), differed significantly among the BMI groups. Compared with the other groups, the underweight group was younger (34.7 ± 14.0, 41.8 ± 13.0, 45.2 ± 12.4, and 45.2 ± 12.3 years, respectively; p < 0.001), had a lower proportion of males (28.5%, 42.4%, 53.9%, and 51.1%; p < 0.001), a smoking rate (17.7%, 23.0%, 35.5%, 30.9%; p < 0.001), and a lower marriage rate (52.3%, 73.2%, 84.2%, 77.7%, p < 0.001). Additionally, the underweight group had higher DAIBD scores (56.7 ± 39.2, 45.8 ± 33.6, 37.3 ± 30.6, and 39.3 ± 34.6; p < 0.001) and a greater proportion of patients with an ESR score of 15 or higher (75.2%, 73.2%, 65.2%, and 59.5%; p < 0.032) (Table 1).
Bonferroni analysis revealed that the underweight group had a significantly lower mean age at diagnosis than the other groups. Specifically, their mean age was 7.1 years lower than that of the normal group (95% CI 3.60–10.58, p < 0.001), 10.5 years lower than that of the overweight group (95% CI 6.36–14.58, p < 0.001), and 10.5 years lower than that of the obese group (95% CI 5.81–15.13, p < 0.001). Additionally, the normal group had a mean age at diagnosis that was 3.4 years lower than that of the overweight group (95% CI 0.09–6.68, p < 0.05).
The underweight group also exhibited significantly higher DAIBD scores than the other groups. Their scores were 10.9 points higher than those of the normal weight group (95% CI 1.690–20.125, p = 0.011), 19.4 points higher than those of the overweight group (95% CI 8.547–30.230, p < 0.001), and 17.4 points higher than those of the obese group (95% CI 5.054–29.733, p = 0.001). No significant differences in DAIBD scores were observed between the normal, overweight, and obese groups.
Bonferroni analysis also showed that the underweight group had a significantly higher proportion of females than the overweight group. Additionally, the underweight group had significantly lower rates of smoking and marriage than the overweight group. The obese group had a significantly lower proportion of high ESR values than the other groups.
Cumulative biologics use according to BMI in patients with intestinal BD
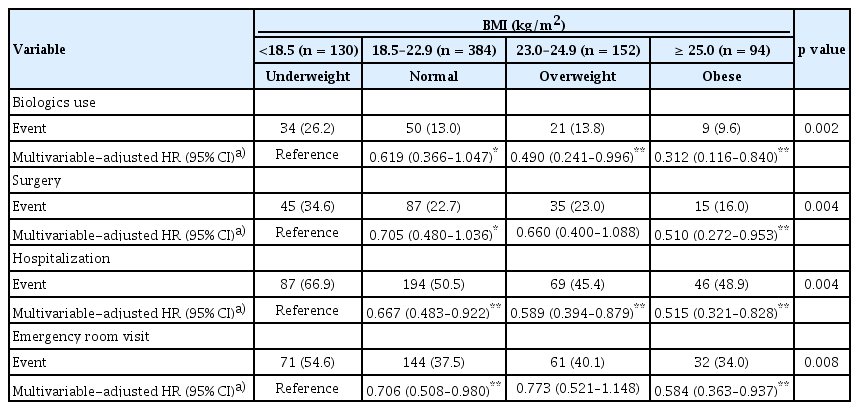
Patients with lower BMI had significantly higher cumulative rates of biologcis use (26.2%, 13.0%, 13.8%, 9.6% % for the underweight, normal, overweight, and obese groups, respectively; p trend = 0.002) (Table 2). The mean times to initiation of first biologics use was 15.0 ± 0.6, 17.7 ± 0.3, 17.9 ± 0.4, and 18.4 ± 0.5 years for the underweight, normal, overweight, and obese groups respectively; with shorter time to biologics initiation observed in the lower BMI group (p < 0.001) (Fig. 1A).
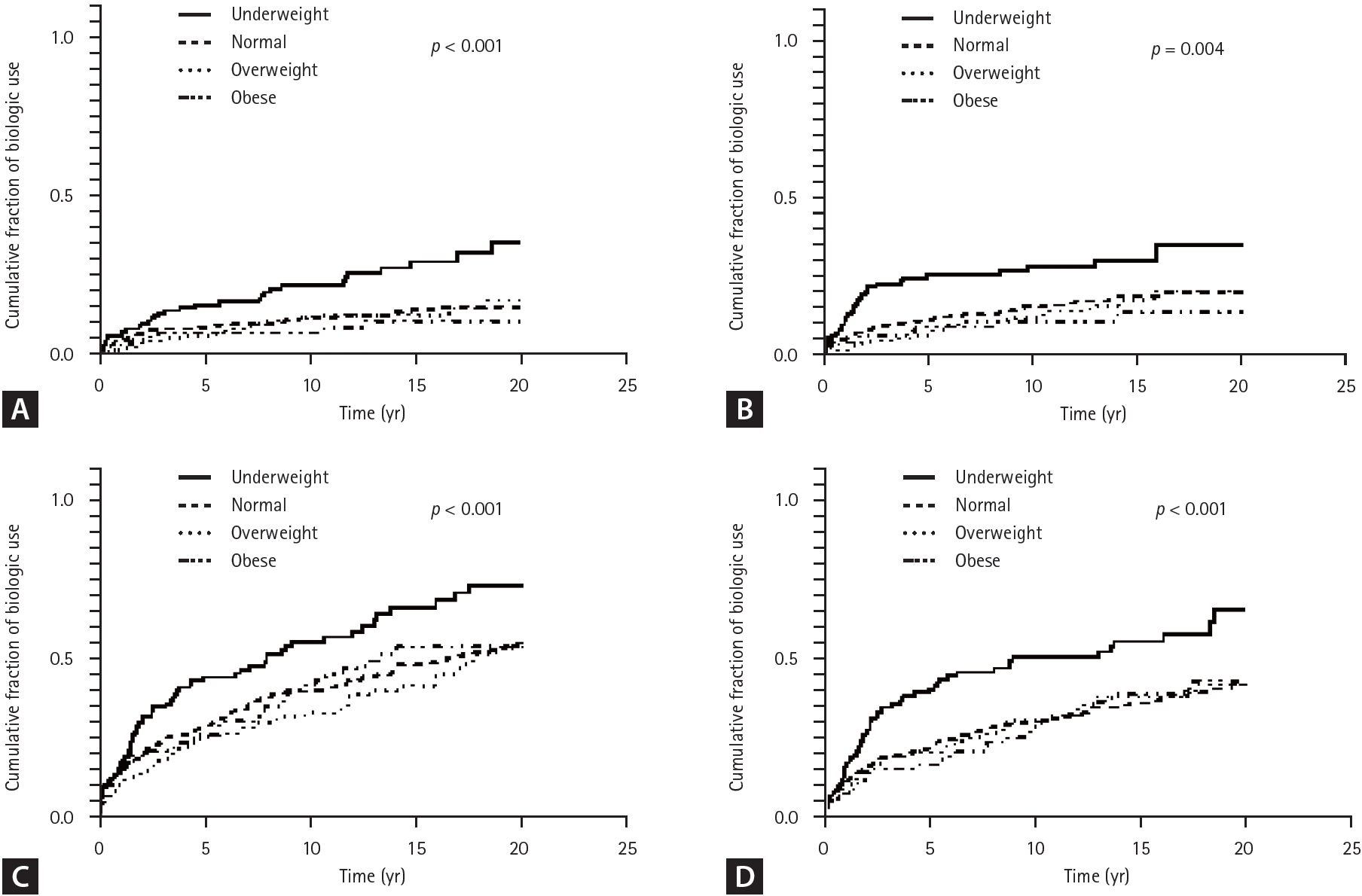
Kaplan–Meier curve for cumulative fraction of (A) biologic use, (B) intestinal BD-related surgery, (C) hospitalization, and (D) emergency room visit by BMI. BD, Behçet’s disease; BMI, body mass index.
In the univariate Cox proportional hazards analysis, several factors—including marital status, DAIBD score, presence of systemic BD, ulcer characteristics (shape, size, depth, and type), C-reactive protein (CRP), and ESR—were significantly associated with biologics use. After adjusting for confounders in the multivariable Cox proportional hazards analysis, future biologics use was negatively associated with the overweight (HR 0.490, 95% CI 0.241–0.996, p = 0.049) and obese groups (HR 0.312, 95% CI 0.116–0.840, p = 0.021) compared with the underweight group (Supplementary Table 1).
Intestinal BD-related surgery according to BMI in patients with intestinal BD
Patients with lower BMI had higher cumulative rates of surgery (34.6%, 22.7%, 23.0%, and 16.0% for the underweight, normal, overweight, and obese groups respectively; p trend = 0.004) (Table 2). The mean times to surgery were 13.3 ± 0.8, 15.6 ± 0.4, 15.9 ± 0.6, and 16.9 ± 0.7 years for the underweight, normal, overweight, and obese groups respectively, with a significantly shorter time to surgery in the lower BMI group (p = 0.004) (Fig. 1B).
In the univariate Cox proportional hazards analysis, several factors—including sex, smoking status, history of appendectomy, DAIBD score, presence of oral ulcers, presence of arthritis, hemoglobin level, CRP, and albumin level—were significantly associated with surgery. After adjusting for confounders in the multivariable Cox proportional hazards analysis, the obese group (HR 0.510, 95% CI 0.272–0.953, p = 0.035) was negatively associated with future intestinal surgery compared with the underweight group (Supplementary Table 2).
Intestinal BD-related hospitalization according to BMI in patients with intestinal BD
Cumulative hospitalization rates decreased with increasing BMI, with rates of 66.9%, 50.5%, 45.4%, and 48.9% for the underweight, normal, overweight, and obese groups, respectively (p trend = 0.004) (Table 2). The mean time to hospitalization was 7.8 ± 0.7, 11.2 ± 0.4, 12.7 ± 0.6, and 11.4 ± 0.9 years for the underweight, normal, overweight, and obese groups respectively, with significantly earlier hospitalization observed in the lower BMI group (p < 0.001) (Fig. 1C).
In the univariate Cox proportional hazards analysis, several factors—including age, marriage status, DAIBD score, presence of eye manifestations, systemic BD, number of ulcers, ulcer depth, hemoglobin level, CRP level, ESR, and albumin—were significantly associated with hospitalization. After adjusting for confounders in the multivariable Cox proportional hazards analysis, the normal (HR 0.667, 95% CI 0.483–0.922, p = 0.014), overweight (HR 0.589, 95% CI 0.394–0.879, p = 0.010), and obese (HR 0.515, 95% CI 0.321–0.828, p = 0.006) groups were negatively associated with future hospitalization compared with the underweight group (Supplementary Table 3).
Intestinal BD-related emergency room visits according to BMI in patients with intestinal BD
Patients in the lower BMI group had higher cumulative rates of emergency room visits, with rates of 54.6%, 37.5%, 40.1%, and 34.0% for the underweight, normal, overweight, and obese groups respectively (p trend = 0.008) (Table 2). The mean time to emergency room visit was 9.9 ± 0.7, 13.4 ± 0.4, 13.3 ± 0.6, and 14.1 ± 0.8 years for the underweight, normal, overweight, and obese groups respectively, with significantly earlier emergency room visits observed earlier in the lower BMI group (p < 0.001) (Fig. 1D).
In the univariate Cox proportional hazards analysis, several factors—including medical aid status, marital status, DAIBD score, presence of skin manifestations, systemic BD, ulcer size and depth, hemoglobin level, CRP level, and albumin level—were significantly associated with emergency room visits. After adjusting for confounders in the multivariable Cox proportional hazards analysis, the normal weight group (HR 0.706, 95% CI 0.508–0.980, p = 0.037) and obese group (HR 0.584, 95% CI 0.363–0.937, p = 0.026) were negatively associated with future emergency room visits compared with the underweight group (Supplementary Table 4).
DISCUSSION
The strength this study lies in its analysis of the relationship between nutritional status and clinical outcomes in a large cohort of patients with intestinal BD, an area that remains poorly researched. This study examined the distribution of weight status in a cohort of 760 patients with intestinal BD, a rare disease. Among them, 130 (17.1%) were underweight, 384 (50.5%) were of normal weight, 152 (20.0%) were overweight, and 94 (12.4%) were obese. The study investigated the time from diagnosis to key clinical events, including surgeries, hospital admissions, emergency room visits, and the use of biologics.
Findings revealed that in intestinal BD, the underweight group experienced significantly poorer clinical outcomes, with higher cumulative rates of biologics use, surgeries, hospitalizations, and emergency room visits compared with the normal, overweight, and obese groups. In contrast, the obese group exhibited no significant differences in clinical outcomes compared with the normal group. These findings suggest that BMI plays a crucial role in the clinical prognosis of intestinal BD, highlighting the importance of individualized patient management based on BMI.
Similar to our study, research on patients with UC found that among 202 patients, 5% were underweight, 55% were normal weight, 26.7% were overweight, and 13.4% were obese. The study suggested that higher BMI may have a favorable impact on UC prognosis, whereas a low BMI is associated with a more severe disease course [22]. A meta-analysis by Hu et al. [23] further demonstrated that obese patients with IBD were significantly less likely to undergo surgery or be hospitalized than their non-obese counterparts.
In Brazil, Lima et al. [24] analyzed 470 patients with IBD, including CD and UC, categorizing them into BMI groups. Among these patients, 194 (41%) were classified as normal weight, 42 (9%) as underweight, 155 (33%) as overweight, and 79 (17%) as obese. The study found that patients with CD were significantly more likely to be underweight than those with UC, and clinical profiles and the frequency of surgical procedures did not differ between the overweight and normal-weight groups.
Consistent with our findings, McKenna et al. [25] examined the impact of BMI on disease severity and postoperative outcomes in patients with CD. Their findings indicated that obese patients exhibited a less severe disease phenotype, characterized by a lower incidence of the fistulizing Crohn’s phenotype and less frequent preoperative immunomodulator use compared with other BMI groups. However, obese patients have a higher rate of postoperative complications.
Our study demonstrated that even after adjusting for DAIBD score and CRP levels, patients with malnutrition and intestinal BD (17.1%) had poorer clinical outcomes than those in the normal, overweight, and obese groups. The chronic inflammatory state in intestinal BD can disrupt the homeostasis of muscle, bone, and adipose tissue metabolism [20,26,27]. Chronic inflammation and malnutrition can lead to impaired mitochondrial activity, decreased energy production, and increased production of reactive oxygen species. Additionally, chronic inflammation and nutritional imbalances can disrupt gut microbial homeostasis, trigger host immune responses, and create a vicious cycle of malnutrition and inflammation [28].
Given these risks, physicians should conduct routine nutritional screening for patients with intestinal BD, recognize the association between malnutrition and disease severity, and implement personalized therapeutic strategies, including nutritional support and/or gradual medical intervention.
Several studies on sarcopenia and obesity in IBD patients have reported findings different from our study. A study by Brown et al. [29] found that in patients with CD, BMI had a non-linear association with disease outcomes, where the greatest risks of composite loss of response, CD-related surgery, and CD-related intestinal resection surgery were observed at the extremes of BMI (underweight and obese categories). A retrospective chart review of patients with UC (1970 and 2010) found that the prevalence of obesity in UC has been increasing and may negatively impact prognosis by increasing the risk of future hospitalization and corticosteroid use [30].
Similarly, a retrospective cohort study of 160 patients with UC treated with biologics found that higher BMI was independently associated with an increased risk of treatment failure, IBD-related surgery, and hospitalization [31]. The differences between our study and these findings may be attributed to variations in body composition between Eastern and Western populations, as well as differences between CD, UC, and intestinal BD.
A study conducted in Korea reported that weight loss was not significantly associated with an increased risk of surgery but noted that patients who experienced weight loss tended to undergo surgery earlier [32]. Meanwhile, a study in Shanghai found that moderate to severe disability is common in patients with CD and is primarily influenced by disease activity and BMI rather than sarcopenia or muscle mass [33]. Given the conflicting results regarding the effects of BMI on IBD, further research is needed to clarify these relationships. Understanding these trends may lead to more personalized treatment approaches that incorporate metabolic health considerations [34].
One of the limitations of this study is the inherent constraint of using BMI as a sole measure of nutritional status. Research on the pathogenesis of IBD suggests that sarcopenia is exacerbated through its connection with the gut-muscle axis [17]. Although BMI is non-invasive and easily obtainable metric, it has limitations in accurately reflecting visceral fat or sarcopenia [35,36]. Additionally, BMI thresholds for defining underweight, normal weight, overweight, and obesity vary across regions and ethnicities, further complicating its applicability in diverse populations. Future research should incorporate assessments of visceral fat and muscle mass using various modalities in combination to improve the accuracy of predicting clinical outcomes in patients with IBD.
Another limitation of this study is that BMI was assessed only at the time of diagnosis, without considering BMI fluctuations over the follow-up period. However, a notable strength of this study is the longitudinal analysis of clinical outcomes throughout the follow-up period, providing valuable insights into the long-term impact of BMI on disease progression.
This study demonstrated that lower BMI in patients with intestinal BD was associated with poorer clinical outcomes, including increased cumulative use of biologics, surgeries, hospital admissions, and emergency room visits. These findings highlight the importance of individualized patient management based on BMI, as it significantly influences disease progression and response to therapy. This underscores the need for future research to incorporate more precise nutritional indicators for improved prognosis and patient care.
Ultimately, personalized treatment strategies, including targeted nutritional interventions, may be essential for optimizing the care of IBD patients. Similar to ongoing research focused on lifestyle and environmental modifications for managing IBD [37,38], individualized treatment approaches—particularly improving nutritional status—are necessary for patients with intestinal BD.
KEY MESSAGE
1. Underweight patients with intestinal BD experienced significantly poorer clinical outcomes, including higher rates of biologics use, surgery, hospitalization, and emergency room visits, than normal, overweight, and obese patients.
2. Routine nutritional screening and personalized treatment strategies, including interventions to optimize nutritional health, are essential for improving prognosis and patient care.
3. Further research is needed to explore the association between nutritional status and clinical outcomes in intestinal BD. Particularly given its high incidence in Korea and similarities to other IBD.
Notes
CRedit authorship contributions
Daye Park: formal analysis, software, writing - original draft, visualization; Jihye Park: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, writing - review & editing, supervision; Soo Jung Park: methodology, resources, investigation, data curation; Jae Jun Park: methodology, resources, investigation, data curation; Tae Il Kim: methodology, resources, investigation, data curation; Jae Hee Cheon: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None