Updates on lung cancer screening for early detection
Article information
Abstract
This review examines the current status and recent progress in lung cancer screening programs, focusing on low-dose computed tomography (LDCT) and emerging liquid biopsy technologies. In Korea, the National Lung Cancer Screening Program has shown promising results in reducing lung cancer mortality since its implementation in 2019. This review discusses the LDCT screening in Korea, including reductions in short-term mortality, increased screening uptake, and enhanced smoking cessation rates. Results from major international trials, including the National Lung Screening Trial, Nederlands–Leuvens Longkanker Screenings Onderzoek trial, and Multicenter Italian Lung Detection studies, demonstrating the efficacy of LDCT in reducing lung cancer mortality, are reviewed. The potential of liquid biopsy as a complement to LDCT is explored, with a focus on multi-cancer early detection technologies. Notable advances include the Circulating Cell-free Genome Atlas study and the Galleri® test, which have shown promise in detecting cancer at early stages through blood-based screening. We also highlight the challenges and limitations of current screening methods, including the need to improve strategies for screening non-smokers and the importance of balancing benefits against risks. As lung cancer screening continues to advance, combining LDCT and liquid biopsy is anticipated to provide more comprehensive and effective early detection strategies.
INTRODUCTION
Lung cancer screening is a multi-tiered approach aimed at reducing mortality through prevention, early detection, and disease management, with low-dose computed tomography (LDCT) being the primary recommended screening tool for high-risk individuals (Table 1). Primary screening (prevention-focused) aims to reduce the risk of developing lung cancer through lifestyle modifications and environmental interventions. Secondary screening (early detection) primarily involves the use of LDCT to detect cancer at an early stage, before symptoms appear. Tertiary screening (monitoring and relapse prevention) focuses on managing diagnosed cases to prevent complications and improve quality of life.
According to the 2021 annual report of the Korea Central Cancer Registry, lung cancer was the third most common cancer, accounting for 11.4% (31,616 cases) of all cancers, with 67% of cases occurring in men and 33% in women [1]. The number of early-stage diagnoses has also increased due to the active implementation of lung cancer screening. According to a report by the Health Insurance Review and Assessment Service in the Republic of Korea (hereafter referred to as Korea), the proportion of lung cancer cases diagnosed at stage I increased significantly from 22.4% in 2013 to 31.4% in 2020 [2,3]. The proportion of locally advanced cases also rose from 23.7% in 2013 to 28.2% in 2020, indicating an increase in cases that can be effectively treated with surgery and perioperative therapy.
This improved lung cancer survival rate is due not only to advances in treatment but also to increased rates of early detection. Based on the findings of the U.S. National Lung Screening Trial (NLST) [4], Korea implemented a nationwide lung cancer screening program in 2019. This program aims to detect early-stage lung cancer using LDCT in high-risk smokers aged 55–74 years with a smoking history of 30 pack-years or more.
In this review, we examine the current status of secondary lung cancer screening programs using LDCT in Korea and abroad and discuss the developmental status and potential applications of liquid biopsy. We hope that the insights provided in this review will deepen our understanding of the advancement of lung cancer screening and contribute to improving early detection rates and survival outcomes.
LUNG CANCER SCREENING IN KOREA
In 2013, the Lung Cancer Screening Recommendation Committee—comprising representatives from the Korean Lung Cancer Society, the Korean Society of Tuberculosis and Respiratory Diseases, the Korean Society of Radiology, the Korean Society of Family Medicine, the Korean Society of Thoracic and Cardiovascular Surgery, and the Korean Society of Preventive Medicine—was formed and began a systematic literature review. Based on the findings of these studies, lung cancer screening recommendations were published in 2015 [5]. It was announced that individuals aged 55–74 years, including current smokers with a history of more than 30 pack-years or former smokers who had quit within the past 15 years, would be considered a high-risk group for lung cancer and should undergo annual screening with LDCT [6].
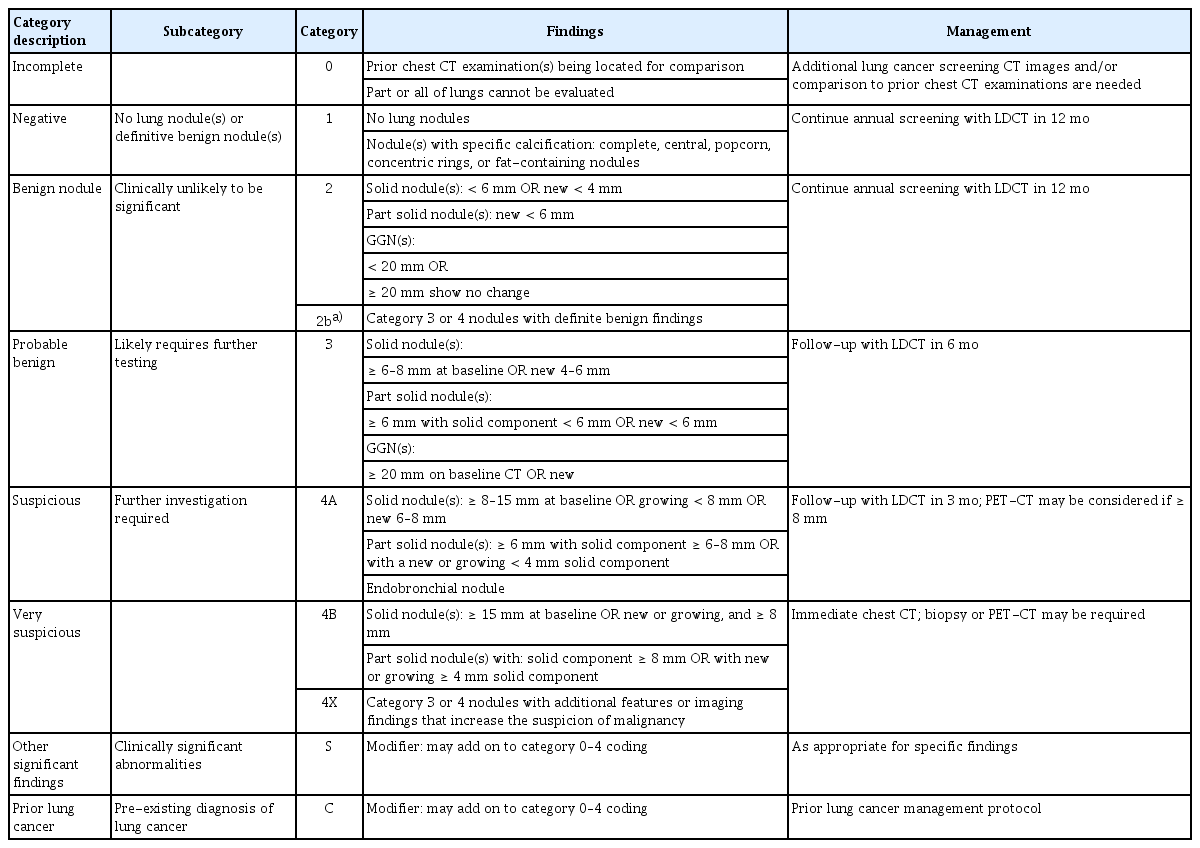
To evaluate the feasibility of implementing a national lung cancer screening program, a pilot project was conducted from 2017 to 2018 [7]. Key screening results are summarized in Table 2. A single LDCT scan was performed on 13,692 individuals nationwide. The average age, smoking history, and proportion of current smokers among the participants were similar to those observed in the NLST. Detected lung nodules were assessed using the Lung Imaging Reporting and Data System (Lung-RADS) version 1.0, a classification system proposed by the American College of Radiology. Although this classification differed from that used in the NLST, a reanalysis of NLST data using Lung- RADS yielded very similar results in terms of positive screening rates, false-positive rates, lung cancer detection rates, and early-stage lung cancer incidence rates. The National Lung Cancer Screening Program (NLCSP) in Korea adopted a modified version of Lung-RADS version 1.0, incorporating adjustments to better reflect the unique epidemiological and clinical characteristics of the Korean population (Table 3).
In November 2018, the National Cancer Control Committee revised its policies to include lung cancer screening as part of the National Cancer Screening Program. LDCT screening began on August 1, 2019, for current smokers aged 54 to 74 years with a smoking history of 30 pack-years or more, and participants have since received LDCT examinations every two years [5]. Unlike the initial screening recommendations, the NLCSP conducts biennial screenings exclusively for current smokers, alternating between individuals born in even-numbered years and those born in odd-numbered years to ensure a rotating schedule every two years. Until 2018, the general health screening questionnaire included a question on the amount smoked but did not capture the duration of smoking cessation for former smokers, making it virtually impossible to identify those who had quit within the past 15 years. To address this limitation, a question on the duration of smoking cessation was added to the health screening questionnaire starting January 1, 2019. Based on this revision, since 2021, individuals who previously participated in the NLCSP remain eligible if they have quit smoking within the past 15 years. Although it is still too early to draw definitive conclusions, the key indicators of the lung cancer screening pilot project aligned with those observed in NLST, suggesting that the NLCSP could significantly reduce both lung cancer–specific and all-cause mortality. Kim et al. [8] reported that the introduction of the NLCSP led to an overall 3.21 percentage point (95% confidence interval [CI]: -4.84 to -1.58) reduction in one-year mortality, including a 2.69 percentage point (95% CI: -4.24 to -1.13) reduction in lung cancer–related mortality, based on National Health Insurance Service claims data from 2018 to 2020.
The screening uptake rate has gradually increased from 33.1% in 2019 to 52.6% in 2022. However, additional efforts are required to further improve participation. Additionally, the continuous smoking cessation rate at six months was only 10.6%, despite smoking cessation counseling provided at the time of screening, indicating a need for innovative strategies to enhance cessation success. Discussions are underway to expand the eligibility criteria for NLCSP. The proposed revisions include: i) Extending the eligible age range to 50–80 years, ii) Lowering the minimum smoking history threshold to ≥ 20 pack-years, iii) Including former smokers with over 30 pack-years of exposure, regardless of the time since cessation. These potential modifications aim to align the program more closely with updated international guidelines, such as those issued by the United States Preventive Services Task Force [9]. If implemented, these changes could significantly broaden the program’s coverage—potentially encompassing up to 37.4% of lung cancer patients in the Korean Lung Cancer Registry, compared to the current 24.3% [10]. However, despite the increasing incidence of lung cancer among never-smokers, extending screening to this group is not currently supported by evidence and would exceed the capacity of the Korean National Health Insurance Service [11].
In high-risk populations, regular LDCT screening through age 74 and long-term follow-up may yield significant reductions in lung cancer mortality. This expectation is supported by the Multicenter Italian Lung Detection (MILD) study, which demonstrated that repeated LDCT screening over a 10-year follow-up period led to a significant reduction in lung cancer–specific mortality. Notably, mortality reduction was not observed at the five-year mark but became more apparent at 10 years, highlighting the potential benefits of extended screening and surveillance for high-risk individuals [12,13].
LUNG CANCER SCREENING USING LDCT IN OTHER COUNTRIES
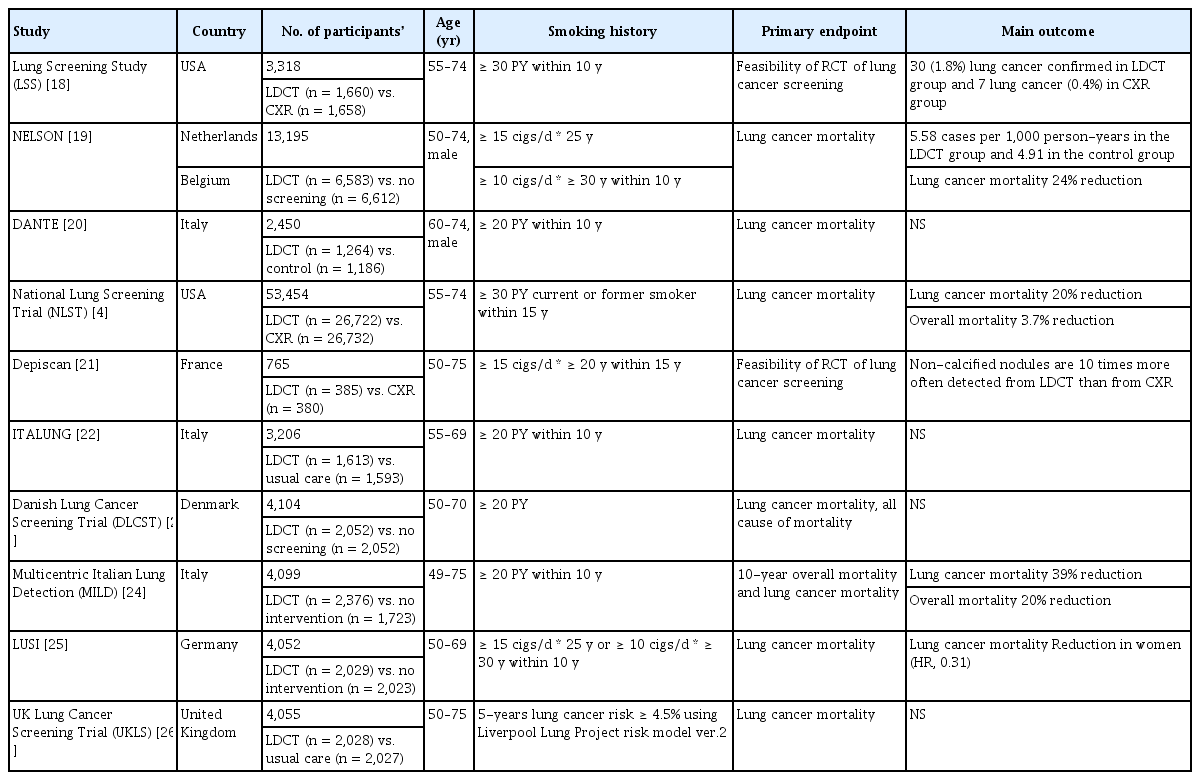
Since the 1950s, when the increasing incidence and risks of lung cancer began to gain attention, active screening studies using chest X-ray and sputum cytology were conducted. However, no significant efficacy was demonstrated [14]. By the late 1990s, several studies began to highlight the usefulness of LDCT for lung cancer screening [15]. One of the key early studies was the Early Lung Cancer Action Project (ELCAP), initiated in 1992 to investigate the effectiveness of annual LDCT screening in high-risk populations [16]. The study included 1,000 asymptomatic volunteers over the age of 60 with a smoking history of at least 10 pack-years. After structured interviews and informed consent, both chest X-ray and LDCT were performed for each participant, and the two modalities were compared in terms of nodule detection rates and the size of non-calcified nodules. The results showed that baseline LDCT identified lung nodules in 233 participants (23.3%), of which 27 cases (2.7%) were malignant. In contrast, chest X-ray detected nodules in only 68 individuals (6.8%), with 7 cases (0.7%) confirmed as malignant. Notably, 23 of the 27 malignant cases detected by LDCT (85.2%) were stage I, whereas only 4 of the 7 malignant cases detected by chest X-ray (57.1%) were stage I. Following the promising outcomes of ELCAP, multiple follow-up studies were conducted to evaluate the utility of LDCT for lung cancer screening. These studies varied in inclusion criteria, such as age range, smoking history, sample size, and follow-up duration (Table 4) [17].
Among these studies, NLST stands out as the largest, enrolling 53,454 participants aged 55–74 years with a smoking history of 30 pack-years or more, including both current smokers and those who had quit within the past 15 years [4]. The NLST demonstrated that, compared to chest X-rays, LDCT reduced lung cancer mortality by 20% and all-cause mortality by 6.7%, providing robust evidence supporting the effectiveness of LDCT for lung cancer screening. The Nederlands–Leuvens Longkanker Screenings Onderzoek trial, conducted in the Netherlands and Belgium, randomized 13,195 men aged 50–74 years to either LDCT screening (at baseline, and years 1, 3, and 5.5) or no screening, with a minimum follow-up duration of 10 years [19]. This trial employed a volumetry-based approach for lung nodule assessment, distinguishing it from other lung cancer screening trials. The screening group had a 90% adherence rate, with 9.2% requiring additional scans and 2.1% referred for suspicious nodules. After 10 years, the incidence of lung cancer was 5.58 cases per 1,000 person-years in the screening group versus 4.91 cases per 1,000 person-years in the control group. Lung cancer mortality was 2.50 deaths per 1,000 person-years in the screening group compared to 3.30 deaths in the control group, yielding a cumulative mortality rate ratio of 0.76 (95% CI, 0.61–0.94; p = 0.01). The MILD trial, conducted in Italy, enrolled 4,099 participants—1,723 in the control group and 2,376 in the LDCT group—and evaluated 10-year outcomes for both all-cause and lung cancer–specific mortality [13]. In the LDCT group, overall mortality was reduced by 20% (hazard ratio [HR], 0.80; 95% CI, 0.62–1.03), and lung cancer mortality was reduced by 39% (HR, 0.61; 95% CI, 0.39–0.95), suggesting that long-term LDCT screening is beneficial not only for early detection but also for reducing both overall and lung cancer–specific mortality.
Field et al. [26] conducted a meta-analysis of nine trials, including those listed in Table 4, and reported that the pooled relative risk of lung cancer mortality in the LDCT group compared to the control group was 0.84 (95% CI, 0.76–0.92), further supporting the efficacy of LDCT in lung cancer screening. According to the National Comprehensive Cancer Network guidelines, prior to 2010, over 90% of lung cancers were attributed to smoking. However, this proportion has since declined to 85–90%. Additionally, a shifting trend in histological subtypes has been observed, with a decrease in squamous cell carcinomas and a gradual increase in adenocarcinomas. As a result, more cancers are now occurring in the peripheral regions of the lungs, often presenting with fewer respiratory symptoms. This evolving landscape underscores the growing importance of LDCTbased lung cancer screening in enabling early detection of asymptomatic cases.
LUNG CANCER SCREENING WITH LIQUID BIOPSY
Liquid biopsy has emerged as a promising tool for lung cancer detection, with potential applications in early screening, disease monitoring, and personalized treatment. Recent studies have explored its use in conjunction with LDCT to enhance diagnostic accuracy and improve patient outcomes. This section discusses how liquid biopsy can be integrated into lung cancer screening programs, complementing LDCT to improve both sensitivity and specificity. This innovative technique analyzes and identifies cancer-associated biomarkers in various body fluids—such as blood, urine, sputum, and saliva—and can aid in early diagnosis, minimal residual disease detection, prognostic assessment, and therapeutic decision-making [27]. Compared to tissue biopsy, liquid biopsy is less invasive and holds potential as an early cancer diagnostic tool by detecting small tumor-derived fragments, including circulating tumor DNA (ctDNA), circulating tumor cells, exosomes, and microRNAs. Significant advancements have been made in these biomarker technologies in recent years [28]. Among these developments, multi-cancer early detection (MCED) technologies— capable of identifying multiple cancer types through a single blood test—have garnered increasing clinical interest. These approaches are currently being evaluated for clinical indications in major cancer types (Table 5).
Serum magnetic resonance spectroscopy metabolomics
In 2021, researchers at Massachusetts General Hospital (affiliated with Harvard Medical School) reported that serum magnetic resonance spectroscopy metabolomics could be used to detect early-stage lung cancer in asymptomatic individuals [29]. Using a predictive model based on serum metabolite profiles, the test demonstrated a sensitivity of 70.4%, specificity of 46.3%, positive predictive value (PPV) of 56.7%, and negative predictive value (NPV) of 61.0%. It also showed potential for predicting five-year survival, highlighting the utility of metabolomics-based approaches in early lung cancer detection.
The GRAIL's trials
In December 2016, the Circulating Cell-free Genome Atlas (CCGA) study was launched to develop an early cancer detection method based on a blood test. This was likely the first major study in early cancer detection conducted by GRAIL (Menlo Park, CA) [30]. The CCGA study is a large-scale, prospective, observational, and case-control study involving approximately 15,000 participants, designed with a five-year longitudinal follow-up. It comprises three substudies aimed at discovering, training, and validating MCED test. Substudy 1 demonstrated that whole genome bisulfite sequencing was the most promising approach for MCED, showing the highest cancer signal detection sensitivity at 98% specificity and the best performance in predicting cancer signal origin (CSO), when compared with whole genome sequencing (WGS) and targeted sequencing [41]. The whole-genome methylation classifier achieved a sensitivity of 39% in the training set and 34% in the validation set at 98% specificity—significantly outperforming most other techniques. These findings confirmed that methylation signatures in ctDNA are effective for both cancer detection and CSO prediction, ultimately influencing GRAIL’s decision to adopt methylation-based methods for early multi-cancer detection. Substudy 2, which included 4,316 participants, incorporated non-cancer samples from the STRIVE study [35], a cohort of 120,000 women undergoing mammography screening. In this phase, specificity reached 99.9% in the training set and 99.3% in the validation set, with sensitivity increasing in later cancer stages. Substudy 3 was a large-scale clinical validation study that included 4,077 participants (2,823 cancer cases and 1,254 non-cancer controls). The MCED blood test evaluated in this phase—marketed as Galleri®—is based on targeted methylation analysis. It demonstrated a specificity of 99.5% (false-positive rate: 0.5%) and an overall sensitivity of 51.5% across all cancer stages, with sensitivity increasing with stage. For 12 pre-specified cancers, the test achieved an overall sensitivity of 76.3% across all stages, including 53.5% for stages I–II and 67.6% for stages I–III. CSO prediction accuracy was 88.7%. The CCGA study validated the MCED platform’s high specificity and low false-positive rate (< 1%) across more than 50 cancer types, with consistent performance in both training and validation datasets.
The PATHFINDER study [32] is a prospective, multicenter, interventional trial involving adults aged 50 years or older, with or without known cancer risk factors. It is notable as the first study in which GRAIL’s MCED test was used to return results—including cancer signal detection and CSO prediction—to healthcare providers in a real-world clinical practice setting. The trial evaluated the implementation of an earlier version of the Galleri® test, assessing how the test results influenced diagnostic decisions and care pathways in a screening population. Between December 2019 and December 2020, 6,662 participants were enrolled, and 6,621 received test results. Cancer signals were detected in 92 participants (1.4%), of which 35 cases (38%) were confirmed as true positives. Among the 6,529 participants without detected cancer signals, the false-negative rate was 1.3%. The test demonstrated a PPV of 38% and a specificity of 99.1%. PPV was higher in participants with additional cancer risk factors (43%) compared to those without (31%). CSO prediction accuracy was 85%. The PATHFINDER study demonstrated the clinical feasibility of the Galleri® test and supported its potential to detect cancer at an early stage and guide diagnostic workflows in routine healthcare settings.
The Guardant Health’s studies
On July 29, 2024, the U.S. Food and Drug Administration approved Guardant Health’s Shield® blood test for colorectal cancer (CRC) screening in adults aged 45 years and older who are at average risk for the disease. This decision was based on findings from the ECLIPSE study, published in the March 2024 issue of the New England Journal of Medicine [40]. The study evaluated circulating cell-free DNA by interrogating genomic alterations, aberrant methylation patterns, and fragmentomic features. The Shield test detected 83% of CRC cases using a non-invasive blood test; however, it missed 17% of cases, primarily those in Stage I. Sensitivity for Stage I CRC was 65%, increasing to 100% for stages II, III, and IV. The test also demonstrated a high overall NPV for CRC of 99.92%. For advanced adenomas (AAs), the Shield test detected 13% of cases, leaving 87% undetected. The overall NPV for AA was 89.86%. These results underscore the Shield test’s potential as a non-invasive diagnostic tool for CRC, particularly for later-stage disease, while also highlighting the need for improved detection of early-stage CRC and AAs. Applying a similar approach in lung cancer, Guardant Health is conducting the SHIELD LUNG study—a large, prospective, multicenter trial enrolling approximately 12,000 participants across 100 centers in the United States and European Union. This study compares the performance of Guardant’s next-generation Shield blood test with LDCT, the current standard screening method [42]. The trial targets high-risk individuals aged 50–80 years. Enrollment of the first participant was announced in January 2022, and the study is expected to enroll nearly 12,000 participants over a 36-month period. Final results have not yet been reported, as the trial is still ongoing.
NHS England Pilot: Targeted Lung Health Check Programme and the SUMMIT study
The UK National Health Service (NHS) England Pilot, known as the Targeted Lung Health Check (TLHC) programme, was launched in 2019 with the goal of improving early lung cancer diagnosis by targeting individuals aged 55–74 years with a history of smoking. Recently, the TLHC programme incorporated a ctDNA blood test, offering it to 10,000 patients by March 2025. This test is being integrated into routine NHS lung cancer care pathways across 80 trusts and is designed to identify tumor-specific genetic mutations within 14 days, enabling patients to avoid unnecessary chemotherapy and gain faster access to targeted therapies. The TLHC programme is offering ctDNA testing to patients with suspected lung cancer, in collaboration with multiple industry partners. Guardant Health, in partnership with The Royal Marsden, offers the Marsden360 test, while Roche Products Ltd, through its affiliate Foundation Medicine Inc., provides a similar assay. In parallel, GRAIL is conducting the NHS-Galleri trial [34], a randomized controlled study involving approximately 140,000 participants, in collaboration with the NHS. This trial aims to evaluate the utility of the Galleri® test for early cancer detection and is expected to conclude in February 2026. Additional ongoing studies—such as PATHFINDER 2 [36], REFLECTION [37], and SUMMIT [30,35]—are designed to refine and expand the clinical applications of the Galleri® test. The NHS England pilot is managed by the NHS Genomic Medicine Service, and through a multi-partner approach, it aims to advance the clinical integration of ctDNA testing in the diagnosis and treatment of lung cancer.
CHALLENGES AND FUTURE DIRECTIONS
While lung cancer screening offers substantial benefits, it is important to acknowledge and address potential risks. These include false positives, which may lead to unnecessary anxiety, additional diagnostic procedures, and potential complications; overdiagnosis, wherein indolent tumors unlikely to cause symptoms during a patient’s lifetime are detected and treated unnecessarily; radiation exposure from repeated LDCT scans; and psychological stress associated with the screening process and the anxiety of waiting for results. These risks should be thoroughly discussed during the shared decision-making process to ensure that patients are fully informed prior to undergoing screening [43]. Balancing the potential benefits of early detection against these risks is particularly crucial for individuals in high-risk groups.
There remains a pressing need to establish more refined and inclusive lung cancer screening strategies—particularly those that address the rising prevalence of lung cancer among never-smokers and women in Korea [44]—as well as to integrate additional modalities that overcome the limitations of LDCT alone.
Recent studies have investigated the combination of liquid biopsy with LDCT for lung cancer screening, aiming to enhance early detection rates and reduce the frequency of false positives. Although LDCT remains the primary recommended screening modality, liquid biopsy shows promise as a complementary tool. Specifically, ctDNA analysis may improve PPV of LDCT [45]. Furthermore, integrating liquid biopsy biomarkers—such as extracellular vesicle long RNA— with imaging features from CT scans has demonstrated impressive diagnostic accuracy, outperforming both single- modal approaches and human experts in certain studies [46]. However, current evidence suggests that liquid biopsy cannot yet serve as a standalone screening modality. Instead, it may be most effectively used as a follow-up test for individuals with positive LDCT findings or for those unable or unwilling to undergo LDCT. Some researchers have also proposed utilizing liquid biopsy as a primary screening tool, followed by confirmatory LDCT in positive cases, potentially increasing screening uptake among high-risk individuals reluctant to undergo imaging. While these multimodal approaches show significant promise, further large-scale, prospective studies are necessary to validate their clinical utility and determine optimal strategies for integration into routine screening practice.
CONCLUSION
Lung cancer screening—primarily through LDCT—offers substantial potential for early detection and improved clinical outcomes. However, several challenges persist, including the need to balance benefits against potential harms and to address population-specific considerations, particularly in Korea. Emerging technologies such as liquid biopsy show promise as complementary tools, with the potential to enhance diagnostic accuracy, reduce false positives, and increase screening acceptability. Moving forward, key priorities should include improving risk stratification models, validating multimodal screening approaches, tailoring strategies to high-risk subpopulations, and enhancing shared decision-making processes between patients and healthcare providers. Advancing both our scientific understanding and the clinical implementation of lung cancer screening is essential for reducing disease burden through earlier detection and more effective, individualized interventions.
Notes
CRedit authorship contributions
Se Hyun Kwak: data curation, formal analysis, writing - original draft; Chi Young Kim: data curation, writing - original draft; Sang Hoon Lee: data curation, formal analysis, writing - original draft; Eun Young Kim: data curation, writing - original draft; Eun Hye Lee: writing - original draft; Yoon Soo Chang: conceptualization, resources, formal analysis, writing - original draft, writing - review & editing, supervision, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by RS-2023-NR076411 awarded to YS Chang.