Altered polyunsaturated fatty acids and oxylipins profile in Behçet’s disease
Article information
Abstract
Background/Aims
Behçet’s disease (BD) is an autoinflammatory disease of unknown etiopathogenesis. Oxylipins i.e., prostaglandins, leukotrienes, lipoxins, resolvins, and protectins are bioactive polyunsaturated fatty acids (PUFAs) derivatives involved in inflammatory response induction and resolution. The study aimed to determine the profile of selected PUFAs and oxylipins and to define a lipidomic signature for BD.
Methods
A case-control study was conducted involving thirty-five patients with BD and thirty-five age and sex-matched healthy individuals as a control group. Selected plasma PUFAs and oxylipins were analyzed using a targeted LC-MS/MS method.
Results
The lipidomic profile was different between the two groups. BD patients showed higher levels of oxylipins deriving from either the n-6-arachidonic acid (i.e., prostaglandin D2, E2, F2α, and 6-keto-F1α, thromboxane B2, leukotriene B4, E4 and F4, and 6-epi and 15-epi-lipoxin A4) or n-3 PUFAs (i.e., 18-hydroxyeicosapentaenoic acid, 7,17-dihydroxy docosapentaenoic acid, protectin X, and resolvin D5), but decreased levels of both n-3 and n-6 PUFAs. Multivariate analyses selected the combination of four mediators, i.e., docosapentaenoic acid, prostaglandin E2, thromboxane B2, and lipoxin A4 as an accurate lipidomic signature for BD.
Conclusions
The profile of PUFAs/oxylipins is altered in BD patients, characterized by increased pro-inflammatory and pro-resolving oxylipins. The findings suggest that oxylipin metabolism might be involved in BD pathophysiology and may represent a therapeutic target for the disease. Further research is required to examine the role of lipid mediators in BD.
INTRODUCTION
Behçet’s disease (BD) is a chronic systemic vasculitis involving all types and sizes of vessels characterized by unpredictable periods of recurrences [1,2]. Mucocutaneous lesions are the most common manifestations, whereas ocular, vascular, and neurological involvement are less common but more serious [1,2]. The mechanisms responsible for the disease and occurrence of flare-ups are unclear. BD patients have an exaggerated inflammatory response driven by a hyperactive innate immune system and a dysregulated adaptive immune system, resulting in a pro-inflammatory mediator excess [3,4]. Hence, controlling the production or responsiveness to pro- and anti-inflammatory factors may be suitable for BD treatment [5,6].
Oxylipins (OxLs) are oxygenated polyunsaturated fatty acids (PUFAs) derivatives with widespread physiological functions [7,8]. The compounds are key mediators and regulators of inflammation, immunity, and hemostasis [8]. Arachidonic acid (AA)-derived prostaglandins (PGs), and leukotrienes (LTs) are pro-inflammatory mediators of the innate immune response [9]. Another genus of OxLs called specialized pro-resolving mediators (SPMs) including lipoxins (LXs), resolvins (RVs), and protectins (PDs) intervene in inflammation resolution [8,10]. SPMs also strengthen host defenses by boosting innate immunity, which helps to resolve inflammation [11,12]. Most studies exploring the inflammatory response in BD focused on cytokines, chemokines, soluble proteins, and immune cells [6,13–16]. Few studies have examined fatty acids/OxLs in BD, focusing on saturated and monounsaturated fatty acids and AA-derived OxLs [17–22], but no previous study examined SPMs in BD. PUFAs and OxLs mediate inflammation initiation, triggering, and resolution [8,10,23]. These mediators affect cardiovascular health while regulating platelet function, hemostasis, thrombosis, and vascular tone [24]. Hence, investigating their clinical implications might have a meaning in BD, a disease associated with inflammation, and a high risk for thrombosis and cardiovascular disease [25]. This study aimed to determine the plasma profile of selected PUFAs and OxLs and define a lipidomic signature for BD. The findings would contribute to clarifying the role of PUFAs/OxLs in BD pathophysiology and providing the basis for controlling the dysregulated immune response.
METHODS
Study design and participants
We conducted a case-control study including BD patients and age and gender-matched healthy controls from March 2022 to July 2023. Diagnosis of BD was confirmed according to the International Study Group criteria [26]. During the study period, BD outpatients presenting to the Department of Internal Medicine at Rabta Hospital (Tunis, Tunisia) for a follow-up visit were invited to participate, and hospital employees/trainees and their relatives were included as a control group. Criteria for non-eligibility were acute/chronic inflammatory illness (apart from BD involvement in patients), renal or hepatic failure, neoplasia, taking dietary supplements or lipid-lowering medications, pregnancy or breastfeeding, and lack of written consent. Patients and disease characteristics were collected from the patient records. Disease activity was evaluated according to Behçet’s disease current activity form (BDCAF) index [27]. A 12-hour fasting venous blood sample was collected from each participant. Blood was centrifuged (within 30 min from venipuncture) in a cold centrifuge (+4°C) at 4,000 rpm for 20 minutes. Plasma was immediately aliquoted and stored at −80°C until analysis (within 6 mo). The Ethics Committee of Rabta Hospital approved the study protocol (approval number: CERB 02/2022), and each participant provided informed written consent. The study adheres to the ethical principles of the Declaration of Helsinki for medical research involving human subjects. It conforms with the guidelines of Strengthening the Reporting of Observational Studies in Epidemiology Statement for observational studies.
Analysis of lipid mediators
Selected plasma PUFAs/OxLs were analyzed by a targeted LC-MS/MS method as previously described [28]. Details of the analysis methods are provided as Supplementary Material.
Statistical analysis
Statistical analyses were performed using the SPSS statistical software version 25 (IBM Corp., Armonk, NY, USA) and Metaboanalyst 5.0 online package (https://www.metaboanalyst.ca). The Kolmogorov–Smirnov test showed that almost all continuous variables including PUFA/OxL values are not normally distributed. Between-group comparisons were achieved by the Student t-test or Mann–Whitney U test for continuous variables and the chi-square test for categorical variables. A two-tailed p value less than 0.05 was considered significant. Values of PUFAs and OxLs were log-transformed and auto-scaled to make them more comparable. Volcano plot analysis (fold change = 1; false discovery rate-adjusted p value [q-value] < 0.1), and multivariate partial least square-discriminant analysis (PLS-DA) were applied to select and classify the mediators that discriminate BD patients from controls. The metabolites with variable importance in the projection (VIP) score value > 1 in PLS-DA are the most valuable mediators discriminating between BD and control groups. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) was used to estimate their accuracy in discriminating BD patients from controls. To define a lipidomic signature for BD, multivariate ROC curve-based exploratory analysis was performed with linear support vector machine (SVM) as the classification method and SVM built-in as the feature ranking method. The analysis generates different algorithms to select the smallest components set that predict a given target with the highest predictive accuracy [29]. In the elected model, metabolites with a selection frequency above 0.8 have good discriminatory power and are included in the biomarker signature. The model performance is evaluated using a cross-validated area under the receiver operating characteristic curve (AUROC). According to Jones and Athanasiou criteria [30], an AUROC > 0.97 indicates an excellent accuracy, 0.93 to 0.96 a “very-good” accuracy, and 0.75 to 0.92 a good accuracy.
RESULTS
Enrolment and main characteristics of the study participants
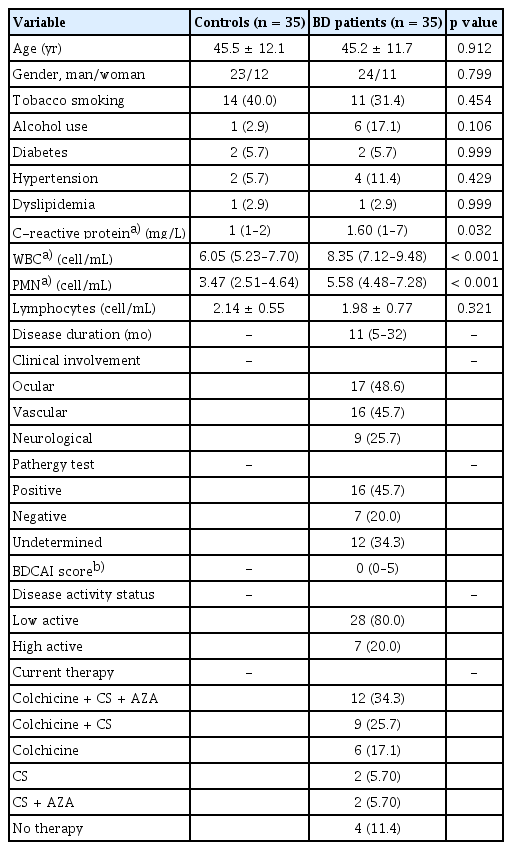
Of 87 BD patients followed in the department, 49 presented for a control visit during the study period. Six patients did not meet the inclusion criteria and eight refused to participate. Of 52 healthy persons, 35 were selected as controls while adjusting with patients for gender, 5-year age class, and social rank. Finally, 35 BD patients and 35 controls were retained for the analysis. BD patients and healthy controls were comparable in terms of age, gender, tobacco smoking, alcohol use, and comorbidities. Considering the BDCAF index, patients were classified as having low-disease activity (n = 28) or high-disease activity (n = 7). Plasma C-reactive protein, white blood cell counts, and polymorphonuclear neutrophil counts were significantly higher in BD patients (Table 1).
Lipid mediators in Behçet’s disease patients and controls
The targeted LC-MS/MS method allowed the quantification of 23 LMs (5 PUFAs and 18 OxLs). Table 2 displays plasma concentrations of PUFAs/OxLs in BD patients and controls. Volcano plot analysis showed differences in lipidomic profile between the two groups (Fig. 1A). Plasma PUFAs (i.e., AA, EPA, DPA, DHA) were lower in BD patients than controls. In contrast, plasma OxLs (i.e., 6-keto-PGF1α, PGE2, PGF2α, PGD2, TXB2, LTB4, LTE4, LTF4, LXA4, 6-epi-LXA4, 15-epi-LXA4, 18-HEPE, 7,17-di-OH-DPA, PDX, RVD5) were higher in patients. The study showed no differences in PUFA/OxL profiles according to clinical involvement, disease activity, and therapy. However, both low and high-disease activity groups showed different profiles from controls. Differences with controls were more frequent in the low-disease activity group. Specifically, n-3-derived SPMs (i.e., 18-HEPE, 7,17-di-OH-DPA, PDX, and RVD5) were only increased in this group (Fig. 1B, C). The PLS-DA 2D score plot shows a clear separation of the clusters of BD patients and controls, with the first (PC1) and the second (PC2) components explaining 38.9% of the model variance (Fig. 2A). The analysis restricted the selection to two PUFAs and eleven OxLs (VIP score > 1) to build a more specific lipidomic profile that discriminates BD patients. The selected model includes DPA, DHA, 6-keto-PGF1α, PGE2, PGD2, TXB2, LTE4, LXA4, 6-epi-LXA4, 15-epi-LXA4, 18-HEPE, 7,17-di-OH-DPA, and PDX as potentially effective discriminating mediators (Fig. 2B). The cross-validation method demonstrated a good power model (accuracy = 0.97, R2 = 0.87, and Q2 = 0.74). The efficacy of individual mediators to discriminate BD was assessed using univariate ROC analysis (Supplementary Table 1).

Plasma concentrations of selected polyunsaturated fatty acids and oxylipins in BD patients and controls

Volcano plot notifying lipid mediators, which were significantly altered compared to controls in (A) whole BD patients, (B) BD patients with low-disease activity, and (C) BD patients with high-disease activity. The volcano plots highlight the mediators with q-values < 0.1 and FC = 1. The metabolites up-regulated, down-regulated, and unchanged in BD patients compared to controls are annotated by red, blue, and gray dots, respectively. The node size means the total number of metabolites in each cluster. The X-axis corresponds to log2 (FC), and the Y-axis corresponds to −log10 (p value). 6-epi-LXA4, 6-epi-lipoxin A4; 6-keto-PGF1a, 6-keto-prostaglandin F1α; 7,17-OH-DPA, 7,17-dihydroxy docosapentaenoic acid; 15-epi-LXA4, 15-epi-lipoxin A4; 18-HEPE, 18-hydroxy eicosapentaenoic; AA, arachidonic acid; BD, Behçet’s disease; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; FC, fold change; LTB4, leukotriene B4; LTE4, leukotriene E4; LTF4, leukotriene F4; LXA4, lipoxin A4; (p), adjusted p value; PDX, protectin DX; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2a, prostaglandin F2a; RVD5, resolvin D5; TXB2, thromboxane B2.

PLS-DA of polyunsaturated fatty acids and oxylipins data from BD patients and CTL. (A) PLS-DA 2D (two-component) score plot displays clustering and class discrimination of BD patients from CTL. (B) VIP plot ranking the first 15 polyunsaturated fatty acids and oxylipins based on their importance in discriminating BD patients from CTL. High VIP scores indicate the metabolites that greatly discriminate BD patients from CTL. The red and blue boxes on the right indicate whether the metabolite is increased or decreased. 6-epi-LXA4, 6-epi-lipoxin A4; 6-ketoPGF1a, 6-keto-prostaglandin F1α; 7,17-OH-DPA, 7,17-hydroxydocosapentaenoic acid; 15-epi-LXA4, 15-epi-lipoxin A4; 18-HEPE, 18-hydroxyeicosa-pentaenoic acid; AA, arachidonic acid; BD, Behçet’s disease; CTL, controls; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; LTB4, leukotriene B4; LTE4, leukotriene E4; LXA4, lipoxin A4; PDX, protectin DX; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PLS-DA, partial least squares discriminant analysis; TXB2, thromboxane B2; VIP, variable importance in projection.
Lipidomic signature of Behçet’s disease
Multivariate ROC curve-based exploratory analysis selected the 7-feature model as the best biomarker model and four mediators (i.e., DPA, PGE2, TXB2, LXB4) to define a lipidomic signature for BD. The four mediators were selected based on their inclusion in the biomarker model with the highest predictive accuracy (88.2%) and good discriminatory power (selection frequency above 0.8). The 7-feature selected model showed “very-good” accuracy with an AUROC of 0.94 (Fig. 3).

Biomarker signature prediction by multivariate ROC curve-based exploratory analysis. (A) ROC curves of six different biomarker models with different numbers of components (2, 3, 5, 7, 10, and 13) and their corresponding AUC with 95 % CI values. (B) The predictive accuracies of the six biomarker models. The red dot specifies the best biomarker model with the highest predictive accuracy. (C) ROC curve of best biomarker model. (D) Rank of features included in the best biomarker model based on their selected frequencies. The red and blue boxes on the right indicate whether the metabolite is increased or decreased in patients. 6-epi-LXA4, 6-epi-lipoxin A4; 6-ketoPGF1α, 6-keto-prostaglandin F1α; 7,17-OH-DPA, 7,17-dihydroxydocosapentaenoic acid; 15-epi-LXA4, 15-epi-lipoxin A4; 18-HEPE, 18-hydroxy eicosapentaenoic acid; AUC, area under the curve; BD, Behçet’s disease; CI, confidence interval; CTL, controls; DPA, docosapentaenoic acid; LTE4, leukotriene E4; LXA4, lipoxin A4; LXB4, lipoxin B4; PDX, protectin DX; PGD2, prostaglandin D2; PGE2, prostaglandin E2; ROC, receiving operating characteristics; TXB2, thromboxane B2.
DISCUSSION
The study showed dissimilar plasma PUFA/OxL profiles between BD patients and controls. The profile in BD patients is characterized by a trend towards a down-regulation of PUFAs and an up-regulation of the OxLs derived from the n-6-AA (i.e., PGs, LTs, LXs) and n-3 PUFAs (i.e., 18-HEPE, 7,17-di-OH-DPA, PDX, RVD5). The disturbed PUFA/OxL profiles in BD suggest that the mediators engage in BD pathophysiology and might represent a therapeutic target for the disease.
Fatty acids are important for immune regulation and their disruption may contribute to some autoimmune and inflammatory diseases [7,31]. Literature data on fatty acid status in BD are scarce and inconclusive. Few studies showed increased saturated and monounsaturated fatty acids [20,21] in BD. PUFAs were either increased [23] or decreased [20]. Zheng et al. [21] found higher free linoleic and AAs in pretreated BD patients, which decreased in post-treated inactive BD patients. The later findings agree with our results of low PUFA levels in post-treated BD patients. The mechanisms responsible for the low levels of PUFA are unknown. The deficit may result from a high conversion into active derivatives including OxLs. Otherwise, it may result from reduced PUFA intake or endogenous biosynthesis. Zheng et al. [21] suggest that BD-specific treatment could influence fatty acid metabolism and that the PUFA profile may indicate BD activity and treatment efficacy. The herein studies showed no significant changes in PUFA profile according to treatment or disease activity.
The pro-inflammatory mediators PGs and LTs were increased in BD patients. This finding is plausible and expected for an autoinflammatory disease like BD. Although most patients had low-disease activity and were taking anti-inflammatory agents, the pro-inflammatory mediators were increased. The treatment could have attenuated a more pronounced increase in the mediators. In our series, four patients were naïve to drugs but had inactive disease, which prevented us from verifying this assumption. However, we could affirm that proinflammatory mechanisms remain active outside BD flares. Besides pro-inflammatory mediators, most pro-resolving SPMs were increased in BD patients. The latter result is somewhat surprising since BD, a chronic inflammatory disease, is supposed to be associated with a deficit in resolution pathways [8,10,23]. Overexpression of SPMs in BD is likely due to neutrophil and mononuclear cell infiltration into tissue lesions [32]. The immune cells release/activate phospholipases, cyclooxygenases, and lipoxygenases, which hydrolyze PUFAs from the cell membrane, and catalyze the synthesis of proinflammatory and pro-resolving OxLs [7]. SPM overexpression in BD patients could be understood as either a failure or a success of inflammation resolution. It may imply a failed attempt to counteract the strong inflammation [33]. Alternatively, as most patients have low-active disease, the increase in SPMs could have resulted in disease attenuation, and transition from a high-active to a low-active disease. Assessing the mediators during the flare-up and remission periods would improve understanding of their role in BD.
Sensitivity analysis by disease activity showed that low- and high-disease activity groups have different lipidomic profiles than controls. The differences mainly relate to n-3-derived SPMs, which only increased in the former group. The findings prove PUFA/OxL metabolism disruption regardless of BD disease activity status. It can be supposed that the SPMs increase has contributed to attenuating the disease in the low-disease activity group. However, this cannot be ascertained due to the disparity in sample size between the two groups. The differences are likely due to a lack of statistical power related to the small sample size in the high-disease activity group.
Research to identify biomarkers specific to BD, including proteins, cytokines, and metabolomic/proteomic/transcriptomic markers reveals a lack of efficacity and applicability in clinical practice [34]. Few metabolomic studies identified panels of biomarkers enclosing PUFAs/OxLs [19,35], but no previous studies had identified PUFA/OxL-based signatures for BD. The current studies suggest that a combination of plasma DPA, LXB4, PGD2, and TXB2 might be an accurate lipidomic signature for BD. Each mediator discriminates BD with at most “good” accuracy (AUC ranging from 0.710 to 0.859) while the combination of the four mediators ensures “very-good” accuracy (AUC, 0.940). However, it should be admitted that this signature lacks specificity and might be irrelevant to everyday practice. The study showed no differences in PUFA/OxL profiles according to clinical involvement, disease activity, or treatment. This may be due to the small sample size in patients’ subgroups; seven had highly active disease and four were not taking therapy. Moreover, multiple combinations of clinical involvement, disease activity, and therapeutic schemes rendered it difficult to detect the proper impact of each condition or combination.
The study shed some light on an unresolved issue by demonstrating altered PUFA/OxL profiles in BD and defining a lipidomic signature for the disease. The findings suggest that changes in PUFA/OxL metabolism could participate in the pathophysiology of BD. The study has limitations that should be acknowledged. It was a single-center study with small sample sizes of patients’ whole group and subgroups. The case-control design prevents ascertaining causality. The study lacks positive control samples for other autoinflammatory diseases. Thus, it cannot be stated that PUFA/OxL changes are specific to BD. Finally, we analyzed a few lipid mediators and did not explore synthesizing/signaling pathways. The findings could apply to Mediterranean populations sharing similar environmental and genetic characteristics with Tunisians but need to be assessed in populations with diverse backgrounds. Further research investigating larger panels of LMs and metabolic pathways while involving control patients with other inflammatory diseases would clarify the implication of OxLs in BD.
In conclusion, the OxL profile in BD patients is characterized by increased pro-inflammatory and pro-resolving mediators. The study suggests that OxL metabolism might engage in BD pathophysiology and serve as a biomarker for the disease. Overexpression of SPMs could reflect either a failure to counteract inflammation or a success in attenuating strong inflammation in BD patients. Further research is needed to understand the role of these bioactive lipids in BD and evaluate their therapeutic potential in the disease.
KEY MESSAGE
1. OxLs are fatty acid derivatives involved in inflammation triggering and resolution.
2. BD associates increased pro-inflammatory and pro-resolving OxLs.
3. Pro-resolving OxLs overexpression fails to resolve inflammation in BD.
Notes
CRedit authorship contributions
Mohamed Kacem Ben Fradj: conceptualization, methodology, investigation, data curation, formal analysis, software, writing - original draft; Ines Naceur: conceptualization, methodology, investigation, data curation, validation, writing - review & editing, visualization, supervision; Emna Talbi: conceptualization, methodology, investigation, data curation, formal analysis, validation, writing - review & editing, visualization, supervision, project administration; Rahma Wada: methodology, investigation, data curation, formal analysis, software, writing - review & editing; Omar Feki: investigation, data curation, formal analysis, software, writing - original draft, writing - review & editing; Monia Smiti-Khanfir: conceptualization, methodology, data curation, formal analysis, validation, writing - review & editing, visualization, supervision; Moncef Feki: conceptualization, methodology, resources, formal analysis, validation, writing - review & editing, visualization, supervision, project administration, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
The work was supported by a grant from the “Ministry of Higher Education and Scientific Research of Tunisia”.