Association between acid-suppressive drugs and risk of psoriasis: retrospective study using Korean National Health Insurance Service-National Sample Cohort
Article information
Abstract
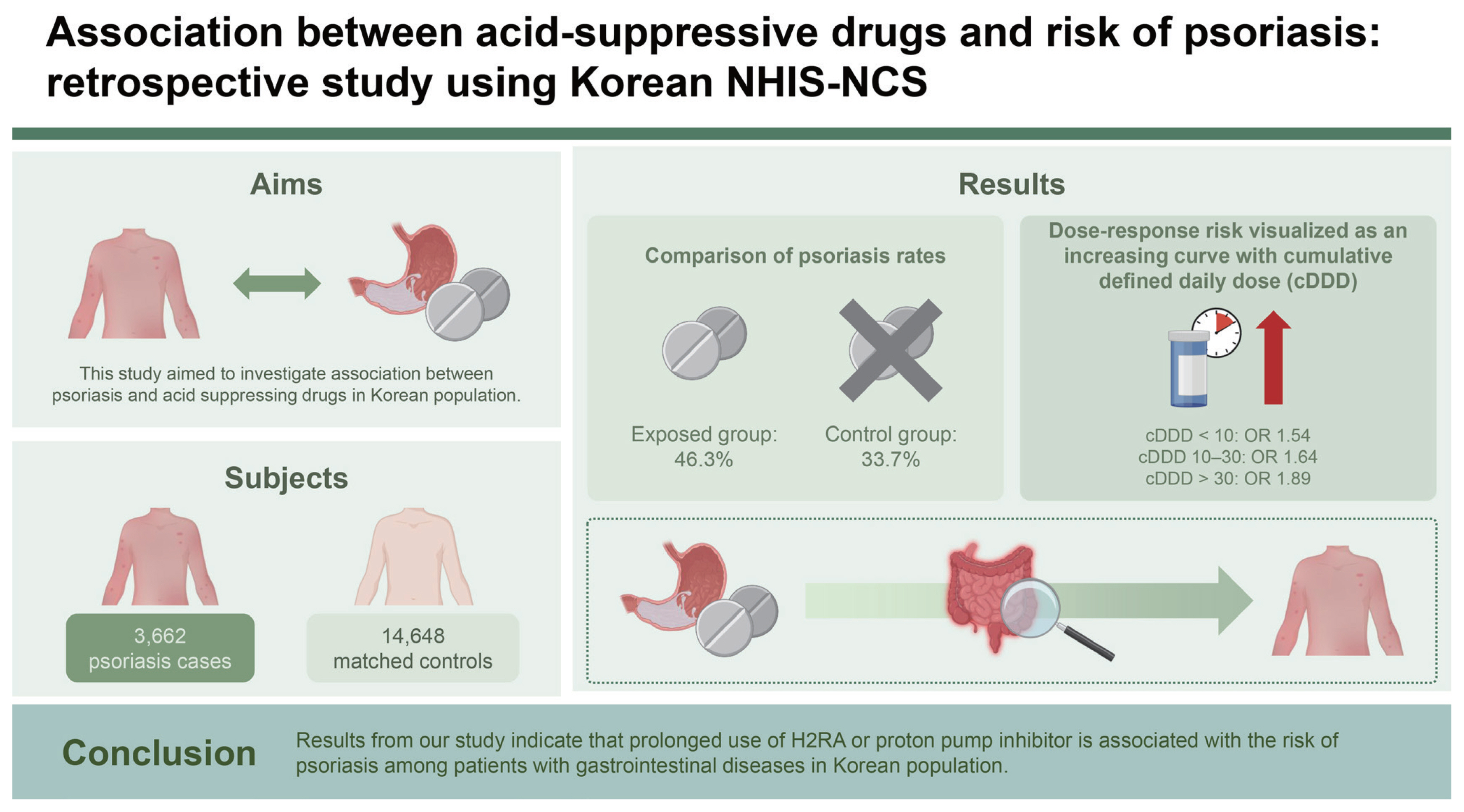
Background/Aims
Psoriasis is a common inflammatory skin disorder following non-specific triggers. Involvement of immune system is widely accepted for pathogenesis studies have demonstrated importance of gut microbiota in pathogenesis of inflammatory skin diseases. Proton pump inhibitor (PPI) and histamine-2 receptor antagonist (H2RA) are acid-suppressive drugs widely used for acid related gastrointestinal diseases, and prolonged use has been associated with altered gut microbiota. This study aimed to investigate association between psoriasis and acid suppressing drugs in Korean population.
Methods
This study was conducted with 3,662 patients diagnosed with psoriasis between 2002 and 2013 in NHIS-NSC. A total of 14,648 controls were matched at 1:4 based on sex, age, and gastrointestinal disease. ORs were estimated to determine the association between acid suppressing drug use and psoriasis.
Results
Our study found a statistically significant association between the prolonged use of acid-suppressive drugs and the development of psoriasis in the Korean population. Specifically, patients with gastrointestinal diseases who used histamine-2 receptor antagonists (H2RA) or proton pump inhibitors (PPI) for extended periods exhibited a higher risk of developing psoriasis. The adjusted odds ratio for psoriasis was 1.89 (95% CI, 1.66–2.17) with long-term use, indicating a clear dose-response relationship.
Conclusions
Results from our study indicate that prolonged use of H2RA or PPI is associated with the risk of psoriasis among patients with gastrointestinal diseases in Korean population. The risk was increased in dose-response trend after adjusting for confounding variables. Clinicians should be aware of risks associated with prolonged use of acid suppressing drugs.
INTRODUCTION
Psoriasis is an inflammatory skin disorder with uncontrolled keratinocyte growth typically presenting as erythematous plaques that are sharply demarcated and covered with whitish scales appearing on scalp, trunk, elbows and knees [1,2]. It is a result of chronic inflammation following non-specific triggers such as scratching, piercing, sunburn, and chemical irritants. It can be exacerbated by medications such as non-steroidal anti-inflammatory drugs [3]. Although its pathogenesis is not clearly understood, genetic susceptibility and involvement of immune system is widely accepted [3–5]. Skin lesions of psoriasis seem to originate from dysregulated interaction between innate and adaptive immunity with cutaneous cells.
Gut microbiota is composed of diverse collection of microorganisms crucial for maintaining healthy immune system. Overgrowth of certain pathognemic organisms or loss of beneficial microbial composition can impact gut immune cells and alteration in production of short-chain fatty acids can have direct impact on systemic inflammation and gut immunity [6–9]. Studies have demonstrated importance of gut microbiota in pathogenesis of inflammatory skin diseases. Scher et al. [10], compared gut microbiota in healthy controls and subjects with psoriasis, and reported less diversity in psoriasis subjects than control, and other research suggests, alteration of gut microbiota as an important part in development of psoriatic disease [11].
Proton pump inhibitor (PPI) and histamine-2 receptor antagonist (H2RA) are acid-suppressive drugs used for acid related gastrointestinal (GI) diseases. Prolonged use of acid-suppressive drugs resulting in long-term reduction of gastric acidity can alter gut microbiota, and studies have reported that use of PPI can increase the risk of allergic diseases including atopic dermatitis and asthma [12,13]. Recent studies have shown dysbiosis after only 4 to 8 weeks of PPI use, and it has can lead to permanent disruption [14,15]. As the gut microbiota can be altered by use of acid-suppressive drugs and gut microbiota has been associated with development of inflammatory systemic diseases including psoriasis, this study aimed to study association between psoriasis and acid-suppressive drugs in Korean population.
METHODS
Data source and study subjects
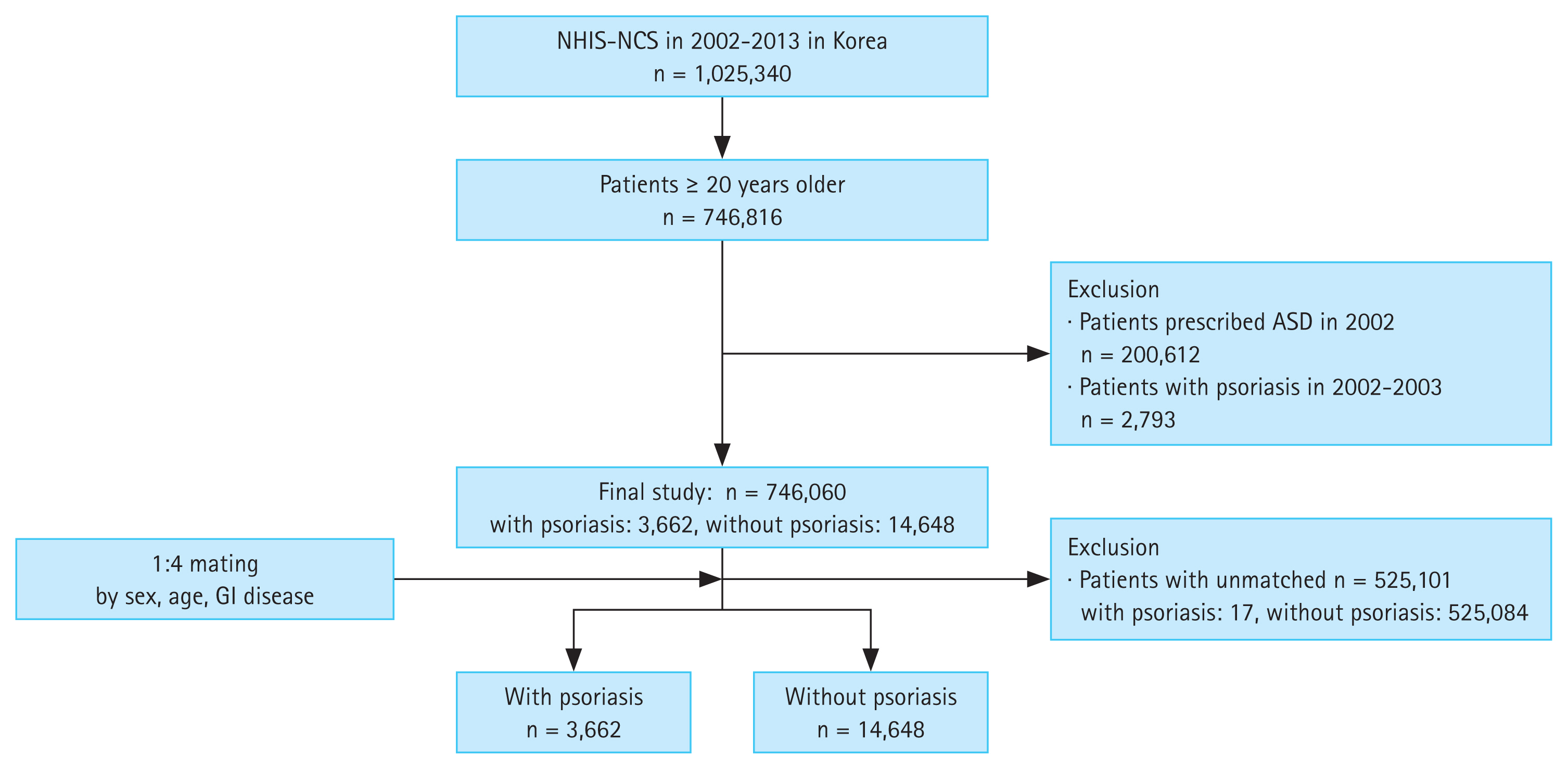
Data was gathered from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC), which is a nationwide cohort database collected from 1 million eligible Korean population who were followed up from 2002 until 2013. It is a nationwide cohort that provides information on patient’s medical history including diagnosis codes, prescribed medications, and information on socio-demographic factors including household income (household income is grouped into 10 separate groups where groups 0, 1, 2, and 3 are categorized as low household income, 4, 5, 6, and 7 are categorized as intermediate, and groups 8, 9, and 10 are categorized as high household income). The case cohort was defined as patients diagnosed with psoriasis (KCD codes L40.0–L40.9) for the first time with prior exposure to acid-suppressive drugs for more than 90 days at least 1 year before diagnosis of psoriasis. The control cohort was defined as subjects who were prescribed with acid-suppressive drugs for more than 90 days, including patients diagnosed with esophageal, gastric, and duodenal diseases (KCD codes K20–K31). From total of 746,816 eligible subjects, 200,612 subjects prescribed with H2RA or PPI in 2002 were excluded and 2,793 patients diagnosed with psoriasis within 1 year after first exposure to acid-suppressive drugs were excluded. After exclusion, analysis included 3,679 subjects in case cohort and 539,732 subjects in control cohort. For each case cohort subject, 4 control cohort subjects were matched using age, sex, and diagnosis of GI diseases. Final analysis included 3,662 subjects in case cohort and 14,648 subjects in control cohort (Fig. 1).
Acid-suppressive drugs and dose
Defined daily dose (DDD) was used for standardization of each medication, which is the assumed average maintenance dose per day for its main indication set by World Health Organization Collaborating Centre for Drug Statistics Methodology [16]. The H2RA included in analysis are cimetidine (800 mg), ranitidine (300 mg), famotidine (40 mg), nizatidine (300 mg), and lafutidine (20 mg). The PPI included in analysis are pantoprazole (40 mg), esomeprazole (30 mg), lansoprazole (30 mg), dexlansoprazole (30 mg), and rabeprazole (20 mg). Cumulative dose equivalent to DDD was counted as 1 and total cumulative DDD (cDDD) was grouped into 0, less than 10, 10–30 and more than 30, where cDDD of 0 was defined as the reference group.
Covariates
Covariates included in the analysis were age (20–29, 30–39, 40–49, 50–59, and ≥ 60), sex (female and male), residence (rural and urban), income (low, medium, and high), and comorbidities which were assessed by Charlson Comorbidity Index (CCI) wherein each comorbid condition were combined to calculate a single score.
Statistical analysis
Cross-tabulation analysis was used for between-group analysis. Odds ratio (OR) with 95% confidence interval (CI) for association between acid-suppressive drugs and development of psoriasis after adjusting for covariates (age, sex, residence, income, and diagnosis of GI diseases) was calculated using logistic regression analysis. A p value of 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS software (version 26.0; IBM Corp., Armonk, NY, USA) and a p value less than 0.05 was considered statistically significant.
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Kangwon National University Hospital (approval no.: KNUH-2024-01-012). This study was conducted in accordance with good clinical practice and principles of the Declaration of Helsinki. Given the retrospective design of the study and the use of anonymized patient data, the requirement for written informed consent was waived by the IRB.
RESULTS
Demographic characteristics
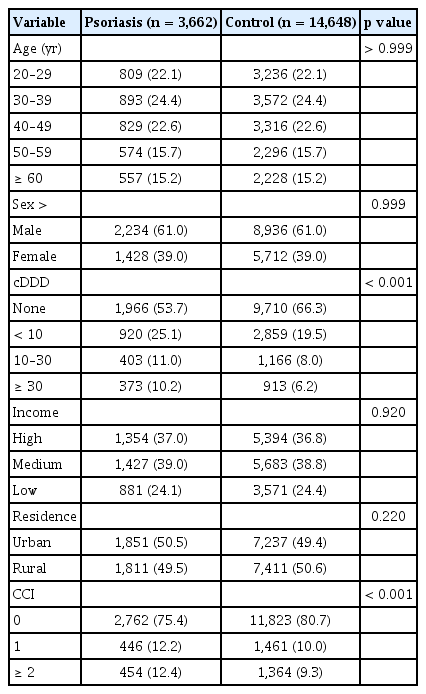
Total of 18,310 subjects with acid-suppressive drugs prescribed for more than 90 days and no prior diagnosis of psoriasis were included in final analysis, among them 3,662 (20.0%) were diagnosed with psoriasis after 1 year since first exposure to acid-suppressive drugs. After matching for age, sex, and diagnosis of GI diseases, total cohort consisted of 11,170 (61.0%) male subjects, 4,045 (22.1%) subjects in age groups 20–29, 4,465 (24.4%) subjects in age groups 30–39, 4,145 (22.6%) subjects in age groups 40–49, 2,870 (15.7%) subjects in age groups 50–59, and 2,785 (15.2%) subjects in age groups older than 60, and total of 9,222 (50.4%) subjects were residing at rural area. Based on household income 6,748 (36.9%) subjects had high income, 7,110 (38.8%) subjects had intermediate income group, and 4,452 (24.3%) subjects had low income. Age, sex, residence, income, and diagnosis of GI diseases were well matched and did not show significant difference between patients with psoriasis and control cohort.
Incidence of psoriasis according to duration of acid-suppressive drugs
The cDDD of acid-suppressive drugs showed different distribution between two groups. Patients with psoriasis were exposed to longer duration of acid-suppressive drugs than control group (25.1% vs. 19.5% respectively in cDDD less than 10, p < 0.001; 11.0% vs. 8.0% respectively in cDDD 10–30, p < 0.001; 10.2% vs. 6.2% respectively in cDDD ≥ 30, p < 0.001), and had greater CCI score than control group (12.4% vs. 9.3% respectively in CCI ≥ 2, p < 0.001). The summary of demographic data is presented in Table 1.
Risk factors of psoriasis
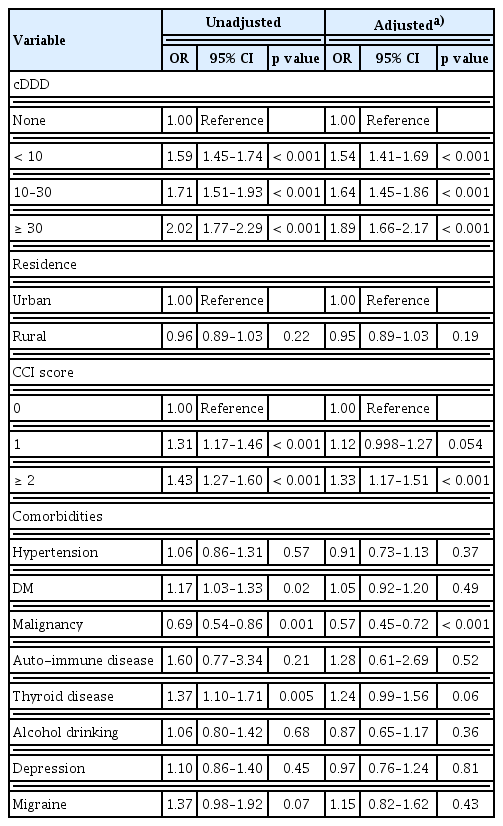
Longer use of acid-suppressive drugs was significantly associated with increased risk of psoriasis. There was significant increase in risk of psoriasis with increasing cDDD (Table 2). Risk of psoriasis for groups using acid-suppressive drugs for cDDD < 10 was 1.59 (95% CI 1.45–1.74, p < 0.001), for groups with cDDD 10–30 was 1.71 (95% CI 1.51–1.93, p < 0.001) and risk for groups with cDDD ≥ 30 was 2.02 (95% CI 1.77–2.30, p < 0.001). After adjusting for possible confounders (residence, CCI, and comorbidities), increased cDDD was still significantly associated with risk of psoriasis. Risk of psoriasis for groups using acid-suppressive drugs for cDDD < 10 was 1.54 (95% CI 1.41–1.69, p < 0.001), for groups with cDDD 10–30 was 1.64 (95% CI 1.45–1.86, p < 0.001) and risk for groups with cDDD ≥ 30 was 1.89 (95% CI 1.66–2.17, p < 0.001). Factors other than acid-suppressive drugs significantly associated with risk of psoriasis based on national cohort was greater CCI score (OR 1.33, 95% CI 1.17–1.51, p < 0.001), and diagnosis of malignant tumors (OR 0.57, 95% CI 0.45–0.72, p < 0.001). Area of residence had no significant effect on risk of psoriasis. Results for risk evaluation are presented in Table 2.
DISCUSSION
This study provides epidemiological evidence for association between acid suppressants and psoriasis in Korean population. We observed that patients with psoriasis were exposed to longer duration of acid-suppressive drugs compared to cohort group, and significant association between prolonged use and increased risk of psoriasis was found in dose-response trend after adjusting for confounding variables (residence, CCI, and comorbidities). The results were similar to our previous study on association between acid-suppressive drugs and risk of rosacea using the same national cohort data [17]. Using national cohort data collected over 10 years, we found other factor associated with risk of psoriasis was CCI score greater than 2 which is in line with other studies that reported hypertension, diabetes, and dyslipidemia are more common in patients with psoriasis than patients without [18].
Acid-suppressive drugs H2RA and PPI are widely used for treatment of acid-related GI diseases, but prolonged use has been associated with adverse effects including respiratory infection, peritonitis, gastric and colorectal cancer, and alteration of gut microbiota [13,19–22]. Proposed mechanisms for such adverse effects include hypergastrinemia induced by altered gastric acidity, hypochlorhydria and related bacterial overgrowth in small bowel and stomach, and alteration in intestinal flora [23–25]. It is well known that PPI is more potent than H2RA, yet alteration of gastric pH itself is a risk factor for change in microbiota, as acidic barrier renders the stomach unfavorable for bacterial survival observed in different studies [26,27].
Epidermal keratinocyte proliferation was believed to be a main mechanism of psoriasis in the past, but past evidence suggested cell-mediated adaptive immune response to play crucial role in development of psoriasis [28]. The connection between drug-induced psoriasis and specific medications highlights the role of the immune system in the pathogenesis of psoriasis. Various drugs such as lithium, beta-blockers, antimalarial drugs, and some antihypertensive medications are known to trigger or exacerbate psoriasis through immune modulation or direct inflammatory pathways [29]. The association between acid-suppressive drugs and psoriasis identified in our study suggests a novel risk factor, different from previously understood mechanisms. Although the results from this study alone are not sufficient to conclude that acid-suppressive drug alone is an absolute risk factor for development of psoriasis as we observed that some patients with psoriasis had no exposure to acid-suppressive drugs and some patients developed psoriasis regardless of medication use. However, greater number of patients exposed to longer duration of acid-suppressive drugs had developed psoriasis which suggests that certain mechanism associated with prolonged use acted as one of multiple factors in development of psoriasis.
Long-term use of acid-suppressive drugs can lead to alterations in gut microbiome. Studies have reported alteration in abundance and diversity of microbiota after using PPI, yet there has been limited research on effect of H2RA in gut microbiota. Shah et al. [30] reported that patients with inflammatory bowel disease exposed to H2RA and PPI were at risk of hospitalization or surgery, in association with alteration of intestinal microbiota and Zhu et al. [13] also reported PPI and H2RA disrupted gut microbiota although the effect was more prominent in PPI which is possibly related to tachyphylaxis of H2RA. Intestinal dysbiosis has been suggested to produce an aberrant immune response in psoriasis as various studies have shown evidence of intestinal microbiota dysbiosis in patients with psoriasis [31–33]. Such changes in microbiota can influence the immune system and contribute to the development of inflammatory skin conditions such as psoriasis and rosacea, emphasizing the importance of “gut-skin axis” [34–40]. Skin and gut share similar important characteristics such as high vascularization, dense innervations, and both are colonized with distinct microbial communities [37]. Also, improvement of skin inflammation after administration of oral prebiotics and probiotics suggest perplexing connection between the gut, brain, and skin [41].
Both PPI and H2RA are acid-suppressive drugs but they are very different in terms of binding site, half life, potency and tachyphylaxis. One of the limitations of this study is that we were not able to separate H2RA and PPI completely because few studies have reported alteration of microbiota with H2RA alone, although the effect may be minimal compared to PPI. Also, there is a possibility that patients are prescribed with both PPI and H2RA depending on underlying comorbidities, as doctors in Korea do not have full access to medication history of all patients. Another limitation is that cohort data used in this study was based on insurance prescription records, which may not reflect the actual clinical environment. There may also be cases with diagnosis of psoriasis that were missed in the data, as diagnosis of psoriasis can only be made by dermatologists. If some patients with suspected psoriasis did not visit dermatology specialist, diagnosis could have been missed.
In conclusion, results from our study indicate that acid-suppressive drugs are associated with the occurrence of psoriasis among patients with GI diseases in Korean population. However, further studies are needed to elucidate the effect of H2RA and PPI separately, and comparing them with patients who have never taken acid-suppressive drugs.
KEY MESSAGE
1. Our study indicates that prolonged use of H2RA or PPI is associated with the risk of psoriasis among patients with GI diseases in Korean population. The risk was increased in dose-response trend after adjusting for confounding variables.
2. Clinicians should be aware of risks associated with prolonged use of acid suppressing drugs.
Notes
Conflicts of interest
The authors disclose no conflicts.
CRedit authorship contributions
Ji Hyun Kim: conceptualization, writing - original draft, writing - review & editing; Joon-hong Min: conceptualization, writing - original draft, writing - review & editing; Young Woo Jo: data curation, formal analysis; Jae Woo Kwon: data curation, writing - review & editing; Young Her: conceptualization, writing - review & editing, funding acquisition
Funding
This study was supported by the Research Grant from Institute of Medical Sciences, Kangwon National University 2024 and by grants from the Basic Science Program through the National Research Foundation