Incidence and risk factors of immune checkpoint inhibitor-induced colitis in Korean patients with cancer
Article information
Abstract
Background/Aims
Immune checkpoint inhibitors (ICIs) are effective in treating cancer. However, various immune-related adverse events (irAEs) have become prevalent, with ICI-induced colitis being the most common gastrointestinal irAE. Thus, we aimed to investigate the incidence and risk factors of ICI-induced colitis in Korean patients with cancer.
Methods
This retrospective study included patients treated with ICIs between October 2015 and June 2022 in two tertiary referral centers in Daegu, Korea. The incidence of ICI-induced colitis was determined using electronic medical records. Risk factors for ICI-induced colitis were identified using univariate and multivariate logistic regression analyses.
Results
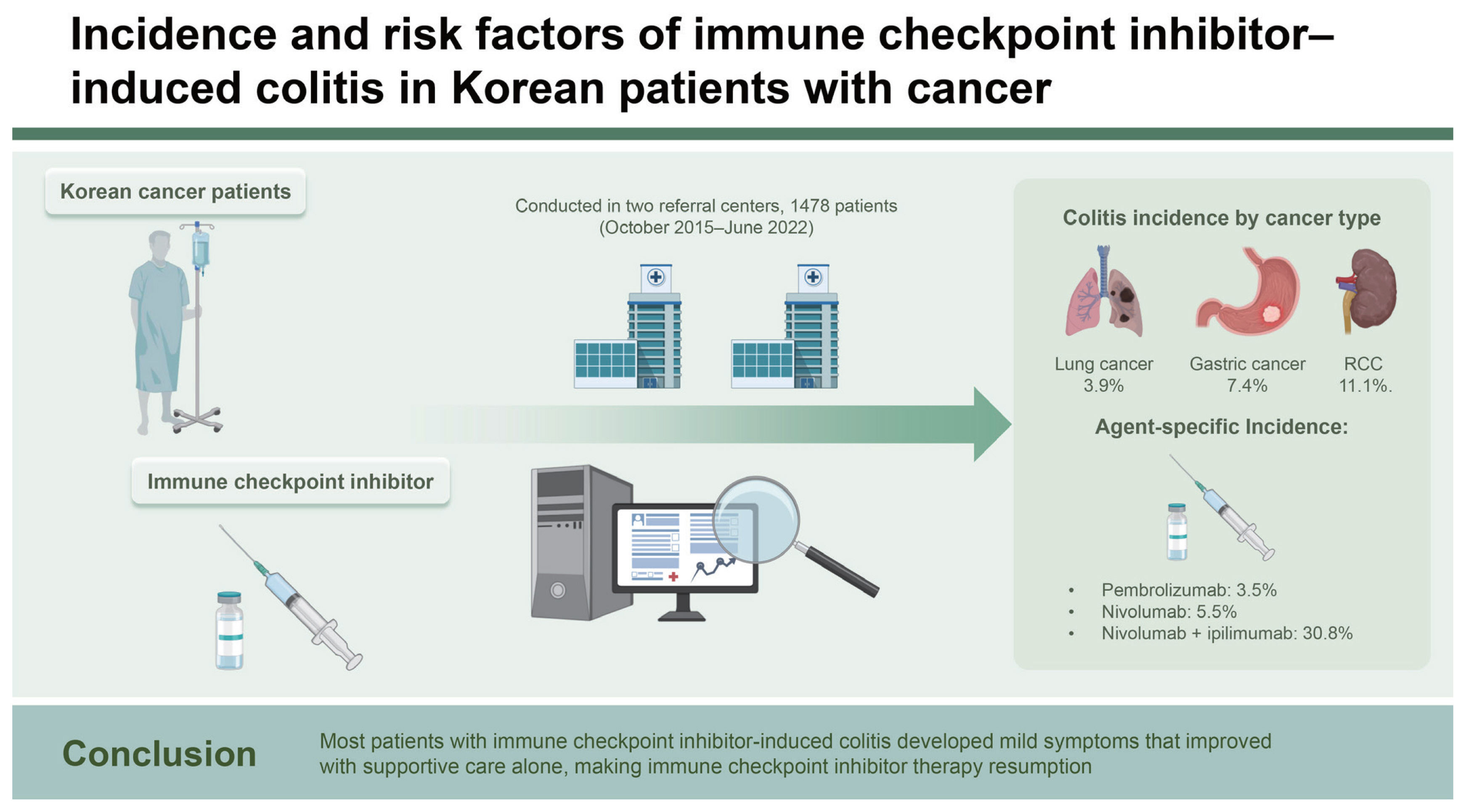
We included 1,478 patients with ICI-treated cancer. The incidence of ICI-induced colitis was 3.5% (n = 52/1,478). Multivariate logistic regression analysis showed that the combination of nivolumab and ipilimumab was a risk factor for ICI-induced colitis (p = 0.006; odds ratio, 9.768; 95% confidence interval, 1.93–49.30).
Conclusions
ICI-induced colitis had an incidence rate of 3.5% and was associated with the combination of nivolumab and ipilimumab. Most patients with ICI-induced colitis developed mild symptoms that improved with supportive care alone, making ICI therapy resumption possible.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) are effective in treating cancer [1]. Particularly, ICIs targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), and its ligand programmed death-ligand 1 (PDL1) have been used in various cancer types [2]. ICI therapy can increase cancer survival rates, expanding the range of ICI-treated cancers [2–4]. The number of patients who respond well to this therapy and are treated with ICIs for a long time is increasing. As ICI utilization increases, various adverse events are also reported. These include colitis, hepatitis, pneumonitis, encephalitis, neuropathy, skin rash, thyroiditis, nephritis, myocarditis, and arthritis [5,6]. ICI-induced colitis is the most common gastrointestinal immune-related adverse event (irAE) [7,8]. Even mild symptoms of ICI-induced colitis can be a problem for patients who need to continue receiving therapy. In severe cases, ICIs must to be stopped as they can lead to life-threatening conditions [9,10]. Specifically, if left untreated, ICI-induced colitis can cause serious complications, such as toxic megacolon and perforation [11,12]. Its incidence was reported to be up to 30%, varying depending on the specific agent, dose, and combination [13,14]. As ICI use becomes widespread, adverse events such as ICI-induced colitis are expected to increase. Currently, the incidence and risk factors of ICI-induced colitis in Korea remain unreported. Therefore, this study aimed to analyze the incidence and risk factors of ICI-induced colitis in Korean patients with ICI-treated cancer.
METHODS
Study design and participants
This retrospective study enrolled patients with ICI-treated cancer at two tertiary referral centers in Daegu, Korea, between October 2015 and June 2022. Patients’ data on ICI usage was collected from the institutions’ electronic medical record charts. Furthermore, the Institutional Review Board of Kyungpook National University Hospital (KNUCH 2022-09-035) approved our study protocol and methods. We specifically included patients who used pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, and ipilimumab at least once between October 2015 and June 2022 and excluded those who participated in clinical trials.
Data collection and definition
Patients’ baseline data such as sex, age, cancer type, prior systemic chemotherapy history, Eastern Cooperative Oncology Group performance status, cancer stage, and colitis development were investigated. The American Society of Clinical Oncology defines ICI-induced diarrhea and colitis according to symptoms without endoscopic evaluation [11]. Increased bowel movement compared with the baseline indicates ICI-induced diarrhea, whereas the presence of abdominal pain, mucus or blood in stools, and fever indicates ICI-induced colitis. Although ICI diarrhea and colitis are clinically distinct terms, diarrhea is a characteristic symptom of ICI colitis, and ICI diarrhea and colitis are used commonly used together. Thus, we defined both as ICI colitis in this study. We considered ICI-induced colitis development according to the chart records of symptoms such as diarrhea, abdominal pain, gastrointestinal bleeding, and colonoscopy or abdominal-pelvic computed tomography (CT) results. Patients with colitis symptoms selected through chart review, were not considered ICI-induced colitis cases if other causes were revealed through colonoscopy, abdominal CT, stool test, or laboratory test [11]. We classified ICI-induced colitis according to the Common Terminology Criteria for Adverse Events (NCI CTCAE v5.0) [15] grade and investigated the number of ICIs used before the onset of ICI-induced colitis, treatment methods, and ICI reuse.
Statistical analysis
Risk factors for ICI-induced colitis were assessed using univariate and multivariate logistic regression analyses. We expressed categorical variables as numbers, and continuous variables as medians and ranges. All statistical data were analyzed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Variables associated with ICI-induced colitis were selected using logistic regression analysis. Significant univariate variables were selected for the multivariate analysis, where the confidence interval (CI) was set at 95%. p values < 0.05 indicated statistical significance.
RESULTS
Out of 1,497 patients with ICI-treated cancer, 1,478 were included in the analysis after excluding duplication or invalid data. Table 1 summarizes their baseline characteristics. Their median age was 67 years (interquartile range, 59–74 yr), with males accounting for 72.3% (n = 1,069). The most common cancer type was lung cancer (n = 909 [61.5%]), followed by malignant melanoma (n = 119 [8.1%]), gastric cancer (n = 94 [6.4%]), bladder cancer (n = 52 [3.5%]), hepatocellular carcinoma (n = 48 [3.2%]), and renal cell carcinoma (RCC; n = 45 [3.0%]). We found that 391 patients (26.5%) used ICIs as the first-line therapy without prior systemic chemotherapy. Of 1,087 patients who received systemic chemotherapy before ICI treatment, 656 (44.4%), 268 (18.1%), 116 (7.8%), and 47 (3.1%) patients were treated with first-, second-, third-, and fourth-line chemotherapy or higher, respectively. Patients taking pembrolizumab accounted for the highest number of individuals receiving ICIs (n = 516 [34.9%]), whereas those taking pembrolizumab plus nivolumab represented the lowest number of people receiving ICIs (n = 1 [0.1%]). Meanwhile, 13 (0.9%) patients with nivolumab plus ipilimumab therapy received ICIs. Until the onset of ICI-induced colitis, the number of ICI administered was 1 in 14 patients, 2 in 8 patients, 3 in 7 patients, and ≥ 4 in 23 patients. Among them, colitis occurred after up to 36 administrations, with a median occurrence number of 3. The incidence of ICI-induced colitis was 3.5% (n = 52/1,478). According to the CTCAE grade of ICI-induced colitis, 40, 7, and 5 patients were classified as grades 1, 2, and 3, respectively. Meanwhile, none had grades 4 and 5 ICI-induced colitis. Regarding ICI-induced colitis management, most patients received only supportive care for symptom relief (n = 43). Steroids were administered to 9 patients, with 1 patient having grade 1, 4 patients having grade 2, and 4 patients having grade 3 ICI-induced colitis. In patients with ICI-induced colitis, 8 patients stopped ICIs after the onset of ICI-induced colitis, whereas 44 remaining patients showed improved symptoms or maintained ICIs with supportive care. Of 8 patients who stopped ICI therapy, 4, 2, and 2 patients were classified as grades 1, 2, and 3, respectively.
The main findings were ulcerative colitis-like findings, with a decrease or loss of vascularity, erythematous and edematous mucosa, and erosions, as well as ulceration in severe cases (Fig. 1). Histological findings showed chronic colitis with erosion, cryptitis, crypt abscess, crypt atrophy, distortion, and lymphoplasmacytic infiltration.

(A) Colonoscopy of a 60-year-old male patient with ICI-induced colitis. Edematous and erythematous mucosa with ulceration was noted in the sigmoid colon. He was diagnosed with lung cancer and treated with pembrolizumab, developing colitis with diarrhea after 15 administrations of pembrolizumab. (B) Colonoscopy of a 65-year-old male patient with ICI-induced colitis. Erythematous friable granular mucosa with decreased vascularity was noted in the descending colon. He was diagnosed with renal cell carcinoma and treated with nivolumab plus ipilimumab, developing colitis with abdominal pain and diarrhea after 5 administrations of nivolumab plus ipilimumab.
The incidence of ICI-induced colitis according to the cancer type was 3.9% (n = 35/909) in lung cancer, 1.7% (n = 2/119) in malignant melanoma, 7.4% (n = 7/94) in gastric cancer, 11.1% (n = 5/45) in RCC, 4.2% (n = 1/24) in cervical cancer, and 4.3% (n = 1/23) in esophageal cancer (Table 2). Based on ICI agents, the incidence was 3.5% (n = 18/516) in pembrolizumab, 5.5% (n = 22/401) in nivolumab, and 30.8% (n = 4/13) in nivolumab plus ipilimumab (Table 3).
Moreover, univariate analysis revealed that factors such as the age of ≥ 70 years (p < 0.001), gastric cancer (p = 0.038), RCC (p = 0.009), and receiving nivolumab (p = 0.014), atezolizumab (p = 0.007), and nivolumab plus ipilimumab (p < 0.001) were significantly associated with ICI-induced colitis. As each checkpoint protein group, CTLA-4 inhibitor (ipilimumab), PD-1 inhibitor (pembrolizumab and nivolumab), and PD-L1 inhibitor (atezolizumab, avelumab, and durvalumab) showed no association with ICI-induced colitis (data not shown). Body mass index, lung cancer, malignant melanoma, esophageal cancer, and pembrolizumab showed no association with ICI-induced colitis (data not shown). In multivariate regression analysis, only the combination of nivolumab and ipilimumab was associated with the risk of developing ICI-induced colitis (p = 0.006; odds ratio, 9.768; 95% CI, 1.93–49.30) (Table 4).
DISCUSSION
This retrospective study investigated the incidence and risk factors of ICI-induced colitis as an adverse event in 1478 patients with ICI-treated cancer at two tertiary referral centers in Daegu, Korea. The incidence rate of ICI-induced colitis was 3.5% (52/1,478), and the combination of nivolumab and ipilimumab was the risk factor for ICI-induced colitis, which is similar to previous studies [12,16,17]. The incidence of ICI-induced colitis was previously reported to be up to 30% [13,14], depending on the specific agent and combination [13]. The cancer type was reported as another risk factor [8], but the result was conflicting. In this study, when the incidence of ICI-induced colitis was analyzed for each cancer type, the incidence was high in a specific cancer, which was not considered a risk factor in the statistical analysis. Concluding that the incidence simply increased depending on the cancer type is impossible, as it might have been caused by the influence of host factors, such as the ICI agents used or underlying diseases. In patients using PD-1/ L1 agents, no difference according to cancer type was reported [18]. In our study, the incidence of ICI-induced colitis in lung cancer, gastric cancer, RCC, cervical cancer, and esophageal cancer was higher than in other cancer types, but these cancer types were not associated with ICI-induced colitis. Previously, ICI-induced colitis was associated with autoimmune diseases, such as rheumatoid disease or inflammatory bowel disease [19]. In this study, there were no cases of rheumatic disease or inflammatory bowel disease among patients considered for ICI colitis. Therefore, the relationship with autoimmune disease could not be confirmed.
The incidence of ICI-induced colitis varied by each ICI agent. In a previous study, the incidence of ICI-induced colitis was 13.6% for the combination of nivolumab plus ipilimumab therapy, 1.4% for PD-1 inhibitor therapy alone (nivolumab or pembrolizumab), 1.0% for atezolizumab alone, and up to 23% for ipilimumab alone therapy [16,18]. In our study, the incidence of ICI-induced colitis according to ICI agents varied from 1.6% to 30.8%. We identified 3.5% (n = 18/516), 5.5% (n = 22/401), 1.6% (n = 8/495), and 30.8% (n = 4/13) incidence in patients receiving pembrolizumab, nivolumab, atezolizumab, and the combination of nivolumab plus ipilimumab therapy, respectively. Unlike in previous reports, no cases of ICI-induced colitis were reported in patients receiving ipilimumab alone therapy.
In previous studies, the incidence of ICI-induced colitis was reported to vary from 1% to up to 30% [13,14], but this wide range of incidence rates might have been reported due to differences in the agents used or cancer types in each study. In this study, most patients with ICI-induced colitis showed mild symptoms. AS not much was known about irAEs in the early days of ICI use, mild symptoms might have been relatively overlooked compared to the diverse and serious symptoms of cancer. In contrast, people have recently started paying attention to even mild symptoms.
Most patients who developed ICI-induced colitis showed mild symptoms of grade 1 (74.1%, n = 40/54). Regarding ICI-induced colitis management, 43 patients (grades 1, 2, and 3 in 39, 3, and 1 patient, respectively) received only supportive care. Nonetheless, most mild symptoms of ICI-induced colitis improved. Of 8 patients who discontinued ICIs because of ICI-induced colitis, 2 patients were classified with grades 1 and 2, respectively, which might have been determined in relation to the patient’s general condition and disease.
ICIs are medications that kill cancer cells by inhibiting the avoidance of the immune system by cancer cells. These agents inhibit PD-1, PD-L1, and CTLA-4, depending on the target immune checkpoint protein. Agents that inhibit PD-1 include nivolumab and pembrolizumab. Agents inhibiting PD-L1 include atezolizumab, avelumab, and durvalumab. Agents that inhibit CTLA-4 include ipilimumab. Nivolumab is mainly used in melanoma, non-small cell lung cancer, stomach cancer, and esophageal cancer. Pembrolizumab is mostly used in melanoma, and non-small cell lung cancer. Atezolizumab is mainly used in urinary epithelial cancer, non-small cell lung cancer, and hepatocellular carcinoma. Avelumab is used in Merkel cell cancer. Durvalumab is used in non-small and small cell lung cancer. Ipilimumab is used in melanoma. Combination therapy with ipilimumab and nivolumab is mainly used in RCC and metastatic melanoma [1,2].
The mechanism of ICI-induced colitis has not been fully established. The use of ICI agents increases the pro-inflammatory pathway and suppresses the anti-inflammatory pathway, resulting in ICI-induced colitis. Other cell types such as macrophages and regulatory T cells are involved. Additionally, changes in the microbiota-integral barrier due to ICI use are considered to have an effect on this [20,21].
This study has limitations due to the retrospective study design. Other causes of colitis symptoms were not sufficiently excluded. In severe cases, a test was performed to determine the cause; however, in many cases, patients with mild colitis symptoms were only symptomatically treated and did not undergo a cause evaluation. Therefore, since most ICI-Induced colitis patients showed mild symptoms, this is considered a limitation of this study.
The use and dosage of CTLA-4 inhibitors, such as ipilimumab, were related to ICI-induced colitis [22–24]. For example, when CTLA-4 inhibitor alone or a combined regimen, such as nivolumab plus ipilimumab, is administered, the incidence of ICI-induced colitis may increase compared with alone use of PD-1 or PD-L1 inhibitor [12,16,17,25]. High dose of CTLA-4 inhibitor is not a risk factor for ICI-induced colitis [22–24]. In our study, judging the relevance of ICI use and dosage was difficult because of the small number of cases. Furthermore, avelumab monotherapy and pembrolizumab plus nivolumab had some cases of ICI-induced colitis, and durvalumab had a relatively small number of cases. Additional limitations include small sample sizes in certain cancer types (10 patients with gallbladder cancer, 8 patients with tongue cancer, and 7 patients with colorectal cancer). Given that the scope of ICI use expands, these cancer types should be investigated further. In a previous study, patients with ICI-induced colitis taking ICIs exhibited favorable effects [8]. However, the present study did not investigate ICI response.
In conclusion, the incidence of ICI-induced colitis was as low as 3.5%. The development of ICI-induced colitis was associated with the combination of nivolumab and ipilimumab. Therefore, caution is required when prescribing nivolumab plus ipilimumab in patients with cancer. Nonetheless, most patients with ICI-induced colitis developed only mild symptoms, which generally improved with supportive care alone, enabling them to resume ICI therapy.
KEY MESSAGE
1. We investigated the incidence and risk factors of ICI-induced colitis in Korean patients with cancer.
2. ICI-induced colitis incidence was as low as 3.5%, and its development was associated with the combination of nivolumab and ipilimumab.
Notes
CRedit authorship contributions
Tae Kyun Kim: methodology, data curation, formal analysis, writing - original draft, visualization; Hyun Seok Lee: conceptualization, methodology, writing - review & editing, visualization, supervision, project administration; Eun Soo Kim: conceptualization, methodology, data curation, supervision, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None