Bariatric surgery for treatment of morbid obesity in adults
Article information
Abstract
Morbid obesity requires active intervention, with treatment options including lifestyle modification, pharmacotherapy, and surgery. As the prevalence of obesity continues to rise in Korea, it is crucial for specialists and general practitioners to have a comprehensive understanding of obesity and its management. Bariatric surgery is the most effective treatment modality for obesity, leading to significant weight loss and metabolic benefits. It involves surgical alterations of normal anatomical structures to improve overall health. Therefore, selecting the appropriate procedure based on the individual characteristics of patients is crucial. This review highlights the two most commonly performed bariatric procedures worldwide, including in Korea: sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB). Furthermore, it provides a comprehensive overview of the surgical techniques involved in SG and RYGB, addresses potential complications, and presents findings from key studies on the weight loss and metabolic outcomes of these surgeries. Additionally, to support clinical application, the review provides outcome data for these procedures based on studies conducted in Korean populations. In addition to SG and RYGB, this review briefly introduces other surgical and endoscopic options, as well as pharmacological treatments that are currently available or may become viable options in the near future.
INTRODUCTION
Obesity is not solely a consequence of lifestyle choices; it is a multifactorial condition arising from the complex interactions of genetic, epigenetic, physiological, behavioral, sociocultural, and environmental factors. These diverse factors contribute to a prolonged imbalance between energy intake and expenditure, eventually leading to obesity [1,2].
Recognized as a chronic disease, obesity is associated with persistent low-grade inflammation and immune dysfunction [3,4]. Prolonged inflammation can potentially disrupt homeostatic mechanisms, contributing to metabolic disorders commonly associated with obesity, although the underlying pathways remain unclear [4–6]. Obesity is also a major risk factor for various chronic conditions, including cardiovascular disease, diabetes, osteoarthritis, and certain cancers [7].
The worldwide prevalence of obesity tripled between 1975 and 2016, affecting approximately 13% of the population aged over 18 years [7]. Similarly, the prevalence of obesity has risen in Korea, increasing from 30.2% in 2012 to 38.4% in 2021. During this period, the prevalence of class III obesity tripled, rising from 0.38 to 1.09% [8]. Obese individuals experience significantly higher health risks, including a 2.6-fold increased risk of type 2 diabetes mellitus (T2DM), 1.2-fold increased risk of myocardial infarction, and 1.1-fold increased risk of ischemic stroke compared to individuals without obesity [9]. Considering these serious medical implications, addressing obesity is not only the responsibility of obesity specialists but should also be a concern for all healthcare providers.
The treatment of morbid obesity includes lifestyle interventions (diet, exercise, and behavioral therapy), pharmacotherapy, and bariatric surgery. Bariatric surgery was first introduced in the 1950s and advanced significantly with the adoption of laparoscopic techniques in the 1980s [10]. Over time, various surgical approaches have been developed, with some discontinued later. Despite this ongoing evolution, bariatric surgery remains highly effective, with its benefits repeatedly demonstrated. These findings suggest that bariatric surgery is still evolving; however, this progression reflects the unique concept behind this procedure, i.e., surgically altering a normal organ system to achieve specific biological outcomes for improved health. Therefore, selecting the most appropriate surgical procedure requires consideration of factors such as the patient’s T2DM status, severity of reflux esophagitis, and risk of gastric cancer. In this review, we explore the characteristics and outcomes of bariatric procedures most commonly performed in Korea.
INDICATIONS AND TYPE OF BARIATRIC SURGERY
Indications for bariatric surgery
Since 2019, the National Health Insurance Service (NHIS) in Korea has covered bariatric surgery for morbid obesity. Health insurance benefits apply to patients with a body mass index (BMI) ≥ 35 kg/m2 or a BMI ≥ 30 kg/m2 with obesity-related comorbidities [11]. Bariatric surgery is also considered for patients with T2DM who have BMI ≥ 27.5 kg/m2 and blood sugar levels that are inadequately controlled with non-surgical treatments [12]. According to international guidelines published in 2022, the indications for bariatric surgery have been expanded to include patients with a BMI ≥ 35 kg/m2 or BMI ≥ 30 kg/m2 with obesity-related comorbidities, in line with Korean guidelines. Additionally, the guidelines suggest that for Asian populations, a BMI ≥ 27.5 kg/m2 may warrant consideration for bariatric surgery, as BMI does not fully reflect individual fat distribution [13]. As a result, it is possible that the criteria for bariatric surgery in Korea may be further expanded in the future.
Types and characteristics of bariatric surgery
Bariatric surgery can be categorized into three main types: restrictive surgery (which significantly reduces the size of the stomach), malabsorptive surgery (where nutrient malabsorption promotes weight loss), and combined surgery (which incorporates both restrictive and malabsorptive elements). To date, six bariatric procedures have dominated the field: jejunoileal bypass (JIB), Roux-en-Y gastric bypass (RYGB), vertical banded gastroplasty, biliopancreatic diversion, adjustable gastric banding, and sleeve gastrectomy (SG) [10]. Since the first report of JIB in 1954, these surgical techniques have seen periods of rise and decline [14]. According to the 2022 global registry report from the International Federation for the Surgery of Obesity and Metabolic Disorders, SG was the most commonly performed primary bariatric procedure, accounting for 63.3% of surgeries, followed by RYGB at 28.8%, based on data from 24 countries [15]. In Korea, bariatric surgery was first performed in 2003 [16]. Since the beginning of national health insurance coverage for bariatric surgery in 2019, the number of procedures has increased significantly—from 543 in 2010 to 2,146 in 2019, and 2,283 in 2021 [17]. In line with global trends, SG (68%) and RYGB (10.9%) are the most commonly performed procedures in Korea [18]. Therefore, this review focuses on these two surgeries.
SG involves a calibrated longitudinal resection, removing approximately two-thirds of the stomach and leaving a gastric volume of 75–100 ml (Fig. 1). Weight loss following SG is primarily due to the restriction of food intake. As previously mentioned, SG is the most commonly performed bariatric procedure worldwide, attributed to its technical simplicity, improved endoscopic access to the upper digestive tract, and a lower risk of malnutrition compared to RYGB. The most serious perioperative complication of SG is leakage along the staple line, with an estimated prevalence of 1% [19]. In the short term, sleeve stenosis, which can lead to chronic vomiting, occurs in 0.5–3.5% of cases [19]. The main long-term complication of SG is gastroesophageal reflux disease (GERD), with a prevalence of 20–30%. GERD is often effectively managed with proton pump inhibitors [20]. Although potassium-competitive acid blockers have not been extensively studied in SG patients, their potential use for treating GERD in this population has been considered, based on their efficacy as an alternative to proton pump inhibitors in the general population [21].
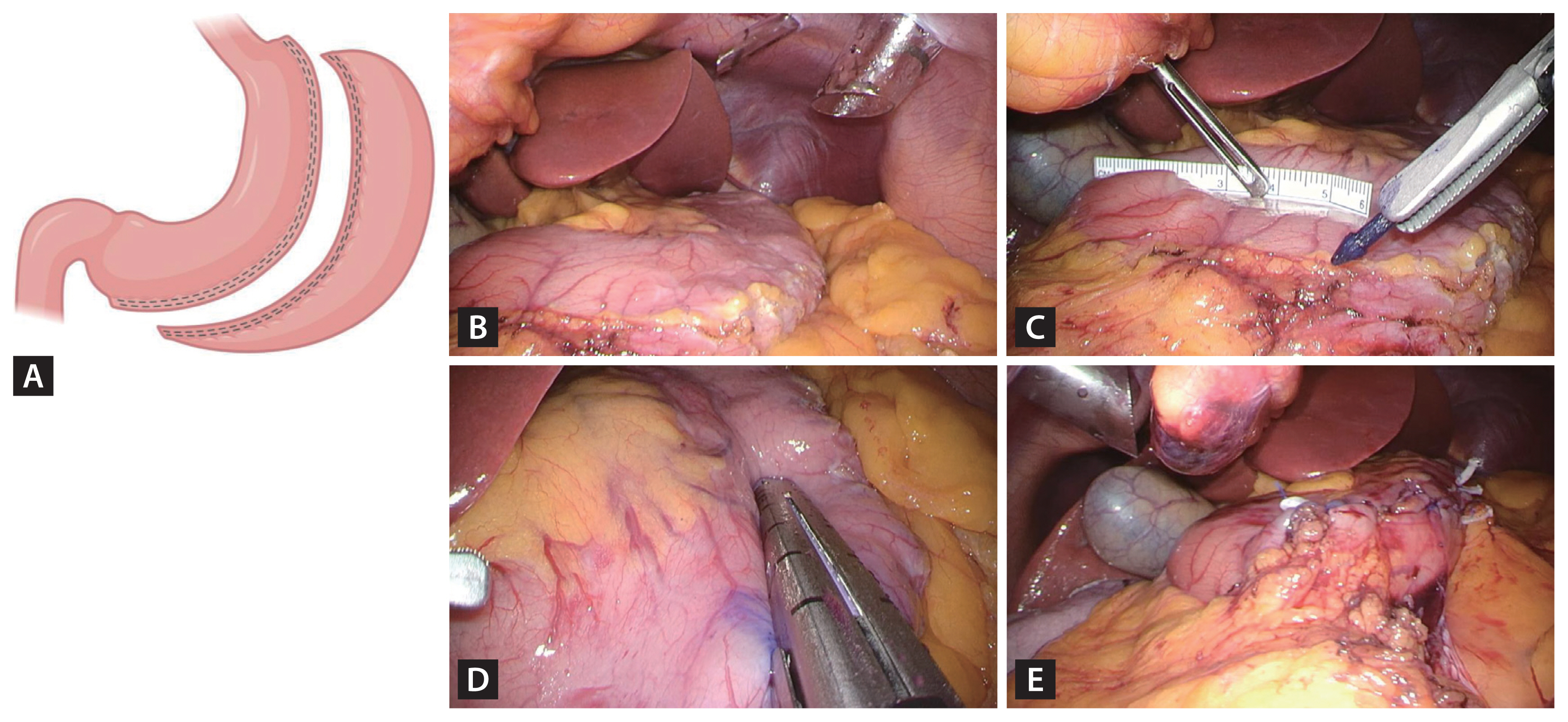
Scheme and surgical images of laparoscopic sleeve gastrectomy. (A) Schematic representation of sleeve gastrectomy. (B) Dissection of the entire greater curvature of the stomach. (C) Maintenance of a 5 cm distance from the pylorus. (D) Division of the stomach from the antrum to the angle of His using sequential stapler firings. (E) Creation of the gastric sleeve. Created with BioRender.com.
RYGB is considered the gold-standard bariatric surgery [22]. Anatomically, RYGB is a combined procedure that involves the creation of a small gastric pouch with a volume of 15–30 mL (Fig. 2). This small pouch restricts food intake, promotes early satiety, and induces malabsorption by bypassing gastrointestinal tract segments. However, RYGB is associated with complications such as dumping syndrome and marginal ulceration. Mechanical complications, including internal hernias, can arise due to anatomical alterations. Nutritional deficiencies, particularly micronutrient deficiencies, may also occur, necessitating ongoing supplementation [23].
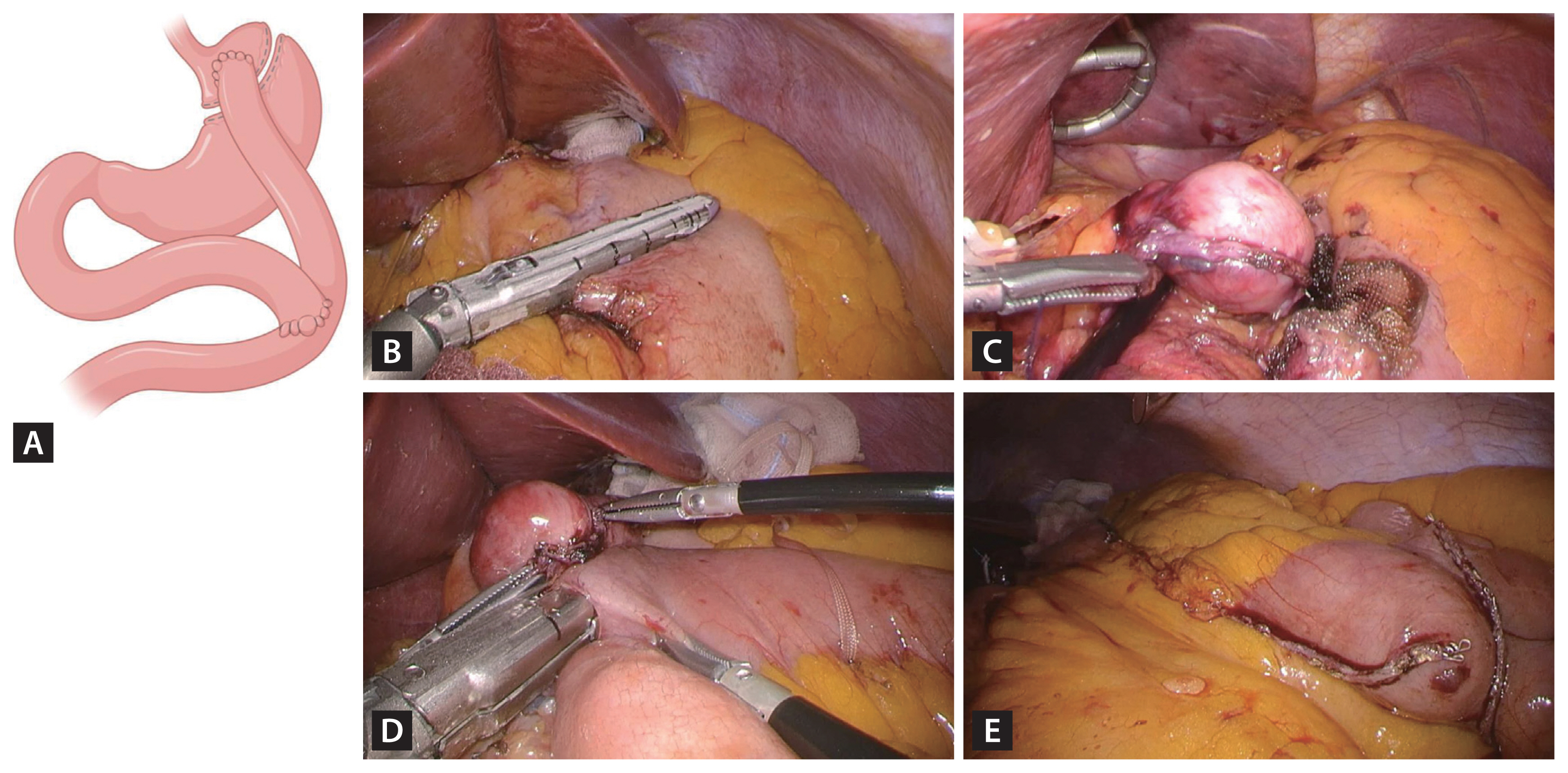
Scheme and surgical images of laparoscopic Roux-en-Y gastric bypass. (A) Schematic representation of Roux-en-Y gastric bypass. (B) Creation of the gastric pouch through horizontal and vertical stapling. (C) Use of a 34–36 Fr bougie to achieve a gastric pouch volume of 25–30 cm3. (D) Performance of a side-to-side gastrojejunostomy with a length < 3 cm. (E) Performance of a side-to-side jejunojejunostomy. Created with BioRender.com.
Endoscopic surveillance of the bypassed stomach after RYGB is particularly challenging, particularly given the high prevalence of gastric cancer in Korea. Although various techniques, including retrograde endoscopy, have been explored to access the remnant stomach, they have proven unreliable in detecting gastric cancer. Resectional RYGB has been proposed as a potential solution, involving the preservation of only the gastric pouch to mitigate the risk of cancer in the remnant stomach. A retrospective study of 20 patients who underwent resectional RYGB demonstrated its feasibility, without a significant increase in surgical complications. These patients also showed better metabolic outcomes compared to those who underwent SG [24]. However, due to the potential need for RYGB reversal in cases of severe complications, such as malnutrition—despite its low incidence—resectional RYGB is not an optimal alternative [25]
A study analyzing data from the 2019–2021 NHIS revealed that the incidence of postoperative complications requiring additional procedures—such as endoscopic or radiologic interventions—or reoperation during the hospital stay was 0.31%. The incidence of complications within 30 days postoperatively was 2.58%, and within 90 days, it increased to 5.76%. The in-hospital mortality rate following bariatric surgery was 0.01% (one patient), with a 90-day mortality rate of 0.05% (four patients) [18]. Detailed information on complications following bariatric surgery in Koreans is anticipated from an upcoming study analyzing the registry data from the Korean Society for Metabolic and Bariatric Surgery.
WEIGHT LOSS FOLLOWING BARIATRIC SURGERY
To comprehend fully the effects of weight loss, it is crucial to understand the commonly used assessment methods. Historically, weight loss outcomes were evaluated using metrics such as the percentage of excess weight loss (%EWL), percentage of excess BMI loss (%EBMIL), and changes in BMI. The %EWL and %EBMIL are calculated based on the weight exceeding the ideal body weight and a BMI of 25 kg/m2, respectively. However, since 2015, the International Bariatric Society has recommended the use of absolute changes in BMI and the percentage of total weight loss (%TWL), as these measures are less influenced by the initial BMI, unlike %EWL [26,27].
Two notable randomized controlled trials (RCTs), the SM-BOSS trial conducted in Switzerland and the SLEEVEPASS trial in Finland, compared %TWL between SG and RYGB. When data from both trials were combined, the results showed %TWL of 28.2% for SG versus 30.8% for RYGB at 1 year, and 23.7% versus 27.2% at 5 years, respectively [28–30]. However, this discrepancy tended to increase over time, with RYGB demonstrating more favorable outcomes for sustained weight loss. In the 10-year follow-up results of the SLEEVEPASS trial, the median %EWL was 43.5% for SG and 50.7% for RYGB, corresponding to 23.4% and 26.9% of %TWL, respectively [31]. A 2021 systematic review reported that, at 1 year post-RYGB, 19 studies with 8,818 patients showed a pooled mean %TWL of 31.9%. For 5-year outcomes from 11 studies involving 3,451 subjects, the pooled mean %TWL was 28.1%. At 10 years, 762 patients from 2 studies had a pooled mean %TWL of 27.8%. For SG, 13 studies with 3,542 patients reported a pooled mean %TWL of 29.5% at 1 year, while 5-year outcomes from 5 studies involving 787 subjects showed a pooled mean of 27.0% [32]. A meta-analysis of 20 RCTs published since 2013 revealed that short-term results (within 3 years post-surgery) showed a %TWL of 28.48 (95% confidence interval [CI] 25.91–31.06) for RYGB and 25.75 (95% CI 19.0–32.51) for SG. Long-term results (3 to 10 years post-surgery) reported %TWL of 25.37 (95% CI 21.87–28.88) for RYGB and 18.67 (95% CI 9.81–27.53) for SG [33].
There are limited studies on the weight loss effects of bariatric surgery reported in Korea. A multicenter retrospective study found that the %TWLs for SG and RYGB were 22.3% and 26.6% at 18 months, and 20.3% and 26.4% at 5 years or beyond, respectively [34,35]. Another nonrandomized prospective study, the KOBESS trial, published in 2021 and involving 64 subjects, demonstrated %TWL at 48 weeks after SG and RYGB of 26.9% and 27.1%, respectively (Table 1) [36].
METABOLIC EFFECTS OF BARIATRIC SURGERY
T2DM
Improved glucose metabolism following bariatric surgery can be attributed to several factors, including reduced caloric intake, weight loss, altered gut physiology, restoration of beta-cell function, and enhanced insulin sensitivity [37]. Among these, an early-stage increase in gut hormones such as glucagon-like peptide-1 (GLP-1) and peptide-YY significantly contribute to energy and glucose metabolism after surgery [38]. Additionally, the exclusion of the foregut has been proposed to optimize glucose metabolism following bariatric procedures [39]. These changes in gut-derived factors align with gut adaptation, a phenomenon that likely plays a key role in driving the impact of bariatric surgery on the remission of T2DM [40,41].
Bariatric surgery has been associated with a T2DM remission rate ranging from 30 to 63% [42]. Over more than 10 years following the surgery, substantial weight loss and improved T2DM outcomes have been consistently demonstrated, distinguishing these results from non-surgical approaches [43,44]. The influential RCT known as the STAMPEDE trial demonstrated that medical therapy supplemented with RYGB or SG was superior to medical therapy alone for the long-term management of T2DM [45]. Recent studies have compared T2DM outcomes between SG and RYGB. In particular, the SLEEVAPASS and SM-BOSS trials found no significant differences in weight loss or diabetes remission between the two procedures at 5 years [28,29]. Comprehensive meta-analyses of observational studies have predominantly indicated higher rates of T2DM remission with RYGB, as compared to SG [46]. Nevertheless, a considerable proportion of patients who initially experience remission— approximately one-third for RYGB and 42% for SG—eventually relapse into T2DM in long-term follow-up studies [47,48]. Considering the current lack of unequivocal superiority between RYGB and SG for T2DM outcomes, the choice between these procedures should primarily consider informed patient preferences in relation to the relative risks associated with each option [49].
In a multicenter retrospective study conducted in Korea, the T2DM remission rate at 18 months post-treatment was 57.1% in the surgical group, which included SG and RYGB, compared to 9.5% in the conventional therapy group, demonstrating a significant difference [35]. In the KOBESS trial, at 48 weeks after surgery, T2DM remission was observed in 46.2% of patients who underwent SG and 50% of those who underwent RYGB, whereas the medical therapy group exhibited a remission rate of 16.7%, demonstrating a significant difference [36] (Table 2). In these Korean studies, T2DM was defined as a fasting glucose level ≥ 126 mg/dL, glycosylated hemoglobin (HbA1c) level ≥ 6.5%, or the use of antidiabetic medication preoperatively. Remission was defined as achieving normal laboratory values without the need for medication.
Recommendations regarding the indication for bariatric surgery in T2DM patients are inconsistent from the Korean Society for the Study of Obesity (KSSO) and the Korean Diabetes Association. Although KSSO suggests a BMI ≥ 27.5 kg/m2, the Korean Diabetes Association recommends a threshold ≥ 30.0 kg/m2 for individuals with uncontrolled T2DM [11,50]. Notably, there are few RCTs that include individuals with a BMI < 30 kg/m2. A meta-analysis evaluated the effects of bariatric surgery on Asian T2DM patients with a BMI < 30 kg/m2, although it acknowledged some limitations in study quality. Despite these limitations, the study has significant clinical implications for Asian populations with lower BMIs. Reductions in HbA1c of 2.38% at 1 year and 1.58% at 2 years were comparable to a prior meta-analysis that predominantly focused on non-Asian populations (which reported a median HbA1c reduction of 2.0%) for patients with a BMI < 30.0 kg/m2 [42,51]. These findings suggest that bariatric surgery could potentially yield comparable HbA1c reductions in individuals with lower BMIs, even though a higher baseline BMI is a well-established predictor of improved T2DM remission rates [52]. Consequently, further clinical studies are needed to understand more fully the impact of bariatric surgery on Asian populations with a BMI < 30.0 kg/m2 [53].
In the context of long-term remission from T2DM, studies primarily focused on Asian populations have consistently demonstrated more favorable outcomes than those predominantly involving non-Asian populations. For instance, a meta-analysis of 37 RCTs revealed a higher T2DM remission rate at 2 years for Asians compared to non-Asians (67.2% vs. 56.3%) [54]. Additionally, another East Asian study involving 463 participants revealed substantial T2DM remission rates of 64.2% at 3 years and 51.4% at 5 years [55].
Dyslipidemia
Among individuals seeking bariatric surgery for severe obesity, approximately 64% have dyslipidemia, characterized by high levels of low-density lipoprotein, elevated triglycerides, and low levels of high-density lipoprotein [56]. Observational studies have revealed that bariatric surgery is associated with short-term (1–2 yr) improvements in dyslipidemia [57]. In a recent meta-analysis comparing RYGB and SG, RYGB exhibited a greater resolution of dyslipidemia at 1 year (risk ratio 0.58, 95% CI 0.46–0.73) and at 5 years (risk ratio 0.68, 95% CI 0.46–0.99) [58]. Another meta-analysis indicated that RYGB shows superior improvement or resolution of dyslipidemia, compared to SG (odds ratio [OR] 1.61, 95% CI 1.05–2.46), although no significant differences were observed after 3 years [59]. In the SLEEVEPASS trial, 47% of patients after SG and 60% after RYGB discontinued dyslipidemia medications at 5 years [29]. However, at 10 years, the remission of dyslipidemia (normal lipid values and no medications) was 19% after SG and 35% after RYGB [31]. Korean studies also demonstrate a similar short-term trend in dyslipidemia remission, as observed in the aforementioned studies (Table 2) [34,35].
Nonalcoholic fatty liver disease (NAFLD)
NAFLD may lead to cardiovascular and hepatic complications, despite its initially benign appearance. Bariatric surgery offers a potential method to slow disease progression and alter the natural course of NAFLD. To understand how bariatric surgery can impede NAFLD progression, it is crucial to recognize the relevant biomarkers and their impacts. Although the liver is the primary organ affected, various biomarkers influence chemical and endocrine functions throughout much of the gastrointestinal system [60]. Key biomarkers include neurotensin (NT) and vitamin D, among others [61,62]. Elevated NT levels are associated with higher rates of NAFLD, cardiovascular disease, T2DM, and obesity. Additionally, vitamin D deficiency or insufficiency is linked to NAFLD progression, in conjunction with elevated fibrinogen and C-reactive protein levels, as well as T2DM [62].
Managing obesity-related NAFLD typically involves achieving a 7–10% weight reduction through lifestyle modifications. However, less than 10% of patients reach this goal within 1 year, and even fewer maintain the weight loss after 5 years [63,64]. Bariatric surgery emerges as a potential option for individuals who are unsuccessful with lifestyle interventions, showing improvements in liver histology, including advanced fibrosis [65]. Despite these benefits, clear guidelines for bariatric surgery in managing NAFLD are lacking [12,13]. The topic remains underexplored, with few prospective studies and no RCTs. In a 5-year prospective study of 1,236 obese patients, the effects of bariatric surgery on liver histology were evaluated [66]. Among the 681 patients who underwent RYGB, 662 (91.2%) had biopsy-confirmed NAFLD. The mean NAFLD activity scores for the RYGB group improved from 2.0 at baseline to 0.7 at both 1 and 5 years. However, only 47.8% and 25.2% of these patients had follow-up biopsies at 1 and 5 years, respectively. The limitations of several published studies include small sample sizes, post-hoc analyses, and heterogeneous patient populations [67]. Meta-analyses have been conducted to address these limitations, yielding consistent findings. Recent meta-analyses from 2015 and 2019 revealed similar improvements in steatosis (50–66%), hepatocyte ballooning (68–76%), and lobular inflammation (50–51%) [68,69].
Cardiovascular and cerebrovascular diseases
Obesity significantly impacts cardiovascular and cerebrovascular disease. In Korea, individuals in their 20s and 30s with obesity experience a 1.7-fold higher risk of myocardial infarction and ischemic stroke compared to those without obesity [9]. Bariatric surgery emerges as a protective procedure for the heart and vasculature [60]. Following bariatric surgery, various biomarkers exhibit positive changes: blood triglyceride and glucose levels decrease, while postprandial adiponectin, GLP-1, insulin, and serum insulin-like growth factor 1 (IGF-1) levels increase. Elevated adiponectin levels are associated with reductions in total fat mass and a decreased risk of atherosclerosis [70]. The postoperative elevation of GLP-1 can reverse obesity-induced endothelial dysfunction, restore the protective properties of high-density lipoprotein, and alleviate insulin resistance [71]. The subsequent reduction in insulin resistance and IGF-1 levels also lowers the risk of increased common carotid intima-media thickness in young obese patients [72].
Studies have shown that bariatric surgery is associated with a significantly lower risk of coronary artery disease or cerebrovascular events at the 5-year follow-up in adults with T2DM and a BMI ≥ 35 kg/m2 (2.1% vs. 4.3%; hazard ratio [HR] 0.60) [73]. Even patients with a history of ischemic heart disease or heart failure who undergo bariatric surgery demonstrate lower rates of major adverse cardiovascular events compared to those who did not undergo bariatric surgery (11.5% vs. 19.6%; HR 0.58) [74].
Systematic reviews have revealed hypertension remission rates of 43–83% 1 year after bariatric surgery, with some evidence suggesting the superiority of RYGB over SG in achieving remission (5-year relative risk 1.26, 95% CI 1.07–1.48). However, blood pressure changes at 5 years may be similar for both procedures [75]. Long-term effects on blood pressure are less established, as approximately 44% of initially successful cases experience hypertension recurrence within a decade [76]. The SLEEVEPASS trial found a similar trend: at baseline, 69% of SG and 73% of RYGB patients were using hypertension medications. After 5 years, 29% of SG and 51% of RYGB patients had discontinued medication [29]. At 10 years, only 8% of SG and 24% of RYGB patients had discontinued medications [31]. In the KOBESS trial, 61.1% of RYGB patients and 59.4% of SG patients experienced hypertension remission at 48 weeks post-surgery [36] (Table 2). Despite these findings, a comprehensive Korean cohort study on the effects of bariatric surgery on hypertension remission is still lacking.
Obstructive sleep apnea (OSA)
OSA is characterized by recurrent episodes of partial or total upper airway obstruction during sleep. Obese individuals are at a higher risk of developing OSA, as fat accumulation in the upper airway can narrow the airway and reduce muscle activity in this area, leading to hypoxemia and apnea symptoms, ultimately resulting in OSA [77]. Each unit increase in BMI is associated with a 1.14-fold higher risk of developing OSA [78]. OSA also leads to hypercapnia, frequent arousals from sleep, and sleep deprivation, which contribute to oxidative stress, inflammatory responses, and activation of the sympathetic nervous system, thereby increasing the risk of metabolic and cardiovascular diseases [79]. OSA severity is assessed using the Apnea-Hypopnea Index (AHI), categorized as follows: < 5 events/h (normal), 5–14.9 events/h (mild), 15–29.9 events/h (moderate), and ≥ 30 events/h (severe) [80].
A recent systematic review of 10 RCTs evaluating anti-obesity medications (including liraglutide, exenatide, zonisamide, dapagliflozin, and phentermine-ER/Topiramate) and bariatric surgery found that weight loss at the study level predicts a change in mean AHI. In particular, a 1% average weight loss is expected to decrease AHI by 0.45 events/h. Although no significant differences were observed in the relationship between weight loss and AHI reduction across studies involving anti-obesity medications versus those involving surgery, bariatric surgery generally led to greater weight loss and a more pronounced decrease in AHI [81].
A study involving 2,310 patients with OSA across 32 studies found that bariatric surgery was associated with a 65% remission rate. OSA remission was defined as the discontinuation of continuous positive airway pressure therapy based on clinical evaluation [82]. According to data from the United Kingdom National Bariatric Surgery Registry, OSA resolution was observed in 64.5% of patients who underwent RYGB and 56.1% of those who underwent SG within 1–2 years after surgery. OSA remission was defined as the cessation of OSA treatment with documented resolution of symptoms [83].
In a study involving 98 Korean bariatric surgery patients, 79 (80.6%) were diagnosed with OSA, with an AHI of 5 events/h or higher, and 52 (53.1%) had moderate-to-severe OSA [79]. A 2014 prospective observational study of 10 Korean patients before and after bariatric surgery demonstrated a significant reduction in the mean AHI, from 51.0 ± 34.2 events/h to 9.3 ± 12.9 events/h. OSA resolution was achieved in five patients (50%) [84].
Polycystic ovarian syndrome (PCOS)
PCOS is the most common hormonal disorder among women of reproductive age, characterized by hyperandrogenism and chronic oligo-anovulation. This syndrome is responsible for female infertility in up to 30% of cases [85]. Additionally, excess weight is a prevalent feature of PCOS, with approximately 60–70% of affected women being either overweight or obese [86]. Even a modest weight loss of 5% of body weight can significantly improve menstrual regularity and fertility in women with PCOS [87].
A prospective nonrandomized trial conducted in China involving 81 patients found that complete remission of PCOS in obese patients is more dependent on the final BMI achieved after weight loss than on the TWL [88]. The BAMBINI trial, a multicenter, open-label RCT, included 80 women (7.5% of whom were Asian) with PCOS, obesity, and oligomenorrhea. This study compared the outcomes of SG and medical therapy (metformin or orlistat) over a 52-week follow-up period. Although the study was conducted before GLP-1 receptor analogues became widely available in the UK National Health Service, the results demonstrated that bariatric surgery was more effective than medical therapy. Women who underwent surgery had a 2.5-fold higher rate of spontaneous, biochemically confirmed ovulations and experienced a restoration of spontaneous menstruation over the 52-week period, indicating an improvement in fertility for these women [89].
Although bariatric surgery has proven effective, the risk of surgical complications remains, which is why it is not considered the first-line treatment for infertility in women with PCOS and obesity. Nevertheless, it has recently been included in the latest international evidence-based guidelines for PCOS [90].
Cancer risk
Obesity is linked to an elevated risk of various cancers, including postmenopausal breast, endometrial, colon, liver, pancreatic, and ovarian cancers [49]. Emerging evidence suggests that bariatric surgery can significantly reduce the incidence of obesity-associated cancers and cancer-related mortality, compared to obese individuals who do not undergo surgery. A meta-analysis involving 635,642 patients reported that bariatric surgery is correlated with a diminished risk across all types of cancer (pooled OR 0.72, 95% CI 0.59–0.87) and a reduced risk of obesity-associated cancer (pooled OR 0.55, 95% CI 0.31–0.96) [91]. Additionally, bariatric surgery can substantially lower overall cancer-related mortality compared to nonsurgical obese controls. A recent retrospective cohort study involving 30,318 patients, with a median follow-up of 6 years, found that obese adults undergoing bariatric surgery exhibited a 32% decreased risk of cancer development and a 48% lower risk of cancer-related death compared to a matched cohort who did not undergo surgery [92].
Mortality
Bariatric surgery consistently demonstrates lower mortality and improved survival rates in large studies. For instance, the SOS study revealed a decrease in adjusted overall mortality among bariatric surgery patients compared to controls over a period of up to 16 years after surgery (HR 0.71, 95% CI 0.54–0.92) [93] Additionally, a meta-analysis involving 170,000 participants indicated an increase of 6.1 years in median life expectancy following bariatric surgery, which is particularly beneficial for those with diabetes [94].
However, it remains uncertain whether all-cause mortality after bariatric surgery aligns with the general population’s rate, matches the new BMI category, or remains persistently elevated due to a higher BMI prior to surgery. A population-based study of 505,258 obese participants found improved standardized mortality rates after bariatric surgery, compared to non-operated obese individuals, yet these rates remained elevated compared to non-obese populations [95]. Numerous patients continue to fall into the overweight or class 1 obesity BMI categories after bariatric surgery, which is associated with the development of obesity-related morbidity and an increased mortality risk [96,97]. Therefore, the elevated mortality observed after bariatric surgery relative to the general population may be attributed to pre-existing conditions prior to the surgery.
ONGOING ADVANCES IN BARIATRIC PROCEDURES
As mentioned previously, no single bariatric procedure is suitable for all patients. Efforts to improve surgical techniques beyond SG and RYGB continue to enhance weight loss and metabolic effects. In 2019, a world consensus meeting on the standardization of bariatric metabolic procedures was held, addressing SG and its 10 variants, as well as RYGB and its four variants [98]. A set of standards derived from peer-reviewed evidence and expert opinion was established to provide a foundation for future research. Among the various procedures, one-anastomosis gastric bypass (OAGB) has been extensively studied. It is the third most commonly performed bariatric surgery worldwide, accounting for 4.1% of cases [15]. In the YOMEGA trial comparing OAGB and RYGB, follow-up results at 2 and 5 years after surgery indicated that OAGB was non-inferior to RYGB in terms of weight loss and metabolic improvement. However, within the first 2 years after surgery, 21.4% of patients undergoing OAGB experienced adverse events related to malabsorptive effects, such as diarrhea, steatorrhea, and nutritional imbalances. At the 5-year follow-up, there were no significant differences in the rate of nutritional complications between the two groups. However, GERD symptoms were significantly more frequent in the OAGB group (42%) compared to the RYGB group (25%), highlighting the need for further studies on this topic [99,100]. OAGB accounts for only 0.2% of bariatric surgeries performed in Korea [18]. Additionally, there are currently no research studies available on Korean patients.
Techniques for treating obesity through less invasive endoscopic approaches, compared to laparoscopic or robotic surgery, are continuously emerging. Since 2024, intragastric balloon placement has become eligible for coverage under the NHIS in Korea. In a recent retrospective study, intragastric balloon placement demonstrated a good efficacy and safety profile in 80 Korean obese patients, achieving a mean %TWL of 10.76% [101]. Although a total of 43.8% of patients experienced complications, no major complications, including mortality, gastric outlet obstruction, or perforation, occurred. The majority of complications were minor, including abdominal pain (54.3%) and nausea and vomiting (68.6%).
Additionally, there is anticipation for the domestic introduction of endoscopic equipment for endoscopic sleeve gastroplasty (ESG). ESG is an innovative endoscopic technique that reduces the size of the gastric reservoir using a full-thickness endoscopic suture device. Although data specific to Koreans are not yet available, studies indicate that the %TWL at 12 months after the procedure is approximately 16%, with the rate of adverse events ranging from 1.5% to 2.3% [102].
PROMISING COMBINATIONS WITH ANTI-OBESITY MEDICATIONS
Since the 2010s, gastrointestinal peptide-based agents, particularly GLP-1 receptor agonists, have emerged as a prominent therapeutic approach for morbid obesity and T2DM [103]. Semaglutide, a novel GLP-1 receptor agonist, has demonstrated substantial weight loss efficacy in clinical trials, with the STEP 1 and STEP 5 trials reporting mean body weight reductions of 14.9% at week 68 and 15.2% at week 104 [104,105]. Despite its effectiveness, studies indicate that discontinuation of semaglutide often leads to significant weight regain, with patients regaining nearly two-thirds of their lost weight within 1 year, suggesting a potential need for long-term use. Additionally, the high cost of semaglutide presents a significant financial burden, and long-term safety data remain limited [103].
Glucose-dependent insulinotropic polypeptide (GIP) is an incretin hormone that influences various metabolic processes, including appetite suppression and fat storage. Dual GIP/GLP-1 receptor agonists combine the effects of GIP and GLP-1 to improve blood glucose control and suppress appetite [103]. Tirzepatide, a dual GIP/GLP-1 receptor agonist, demonstrated a 22.5% reduction in body weight in the SURMOUNT-1 trial, positioning it as one of the most effective agents with generally mild to moderate adverse events [106]. Although evidence is still limited, it can be inferred that tirzepatide may share similar limitations with semaglutide.
Although bariatric surgery remains a highly effective treatment option, it carries a greater risk of complications, and there is currently no optimal treatment available for postoperative weight regain. The combination of new-generation anti-obesity medications with bariatric procedures could potentially yield synergistic benefits. Recently, several retrospective studies have explored the effects of semaglutide following bariatric surgery. A study involving 50 patients who experienced weight regain after surgery found that two-thirds of the regained weight could be safely lost with GLP-1 receptor agonists (liraglutide or semaglutide) [107]. Moreover, the administration of semaglutide at a dose of 2.4 mg/week demonstrated comparable weight loss effects in patients with and without a history of bariatric surgery. In particular, semaglutide treatment resulted in a significant 9.8% weight loss in the bariatric surgery group, with no significant differences in weight loss between the surgery group (9.8%) and the non-surgery group (8.7%) [108].
Conversely, patients who initially used anti-obesity medications and experienced weight loss along with improvements in comorbidities may be more inclined to consider bariatric surgery as a subsequent treatment option, given its long-term efficacy and potential cost benefits. However, further evidence is needed to confirm this possibility.
CONCLUSION
Obesity requires active treatment, and its prevalence has been continuously increasing in Korea. Bariatric surgery is one of the most effective treatments for obesity and its associated comorbidities. This surgical intervention involves the manipulation of normal organ systems to achieve biological outcomes that offer potential health benefits, necessitating the customization of procedures based on the patient’s condition. Worldwide, including in Korea, SG and RYGB are the most commonly performed bariatric surgeries. The approximate %TWL for SG is 26–28% at 1 year after surgery and 23–27% at 5 years or beyond. Furthermore, bariatric surgery has been shown to improve obesity-related comorbidities substantially, including T2DM, dyslipidemia, cardiovascular and cerebrovascular diseases, OSA, and PCOS, due to its metabolic effects. Additionally, bariatric surgery is associated with a reduced cancer incidence and mortality rates. Considering that GERD is a common postoperative complication after SG, careful consideration should be given to patients with preoperative GERD. The approximate %TWL for RYGB is 26–30% at 1 year after surgery and 26–28% at 5 years or beyond. Given that endoscopic surveillance of the remnant stomach is almost impossible, RYGB should be applied with caution, particularly for patients with risk factors for gastric cancer, including family history. Bariatric surgery is continuously advancing, and endoscopic approaches to obesity are also being introduced to clinical practice. In the near future, the synergistic effects of various modalities for obesity treatment, including surgical and endoscopic procedures as well as medications, are expected to enhance weight loss, improve metabolic effects, and prolong the duration of these benefits.
Acknowledgments
Scheme of sleeve gastrectomy and Roux-en-Y gastric bypass in figure 1 and 2 is created with BioRender.com.
Notes
CRedit authorship contributions
Ki Bum Park: conceptualization, methodology, data curation, writing - original draft; Kyong-Hwa Jun: writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by the Korean Gastric Cancer Association (Nos. KGCA042022B1 and KGCA2022IC5) and the Clinical Research Invigoration Project of St. Vincent’s Hospital, the Catholic University of Korea (VC22DIST0078). The study sponsors were not involved in the study design, analysis, data interpretation, report writing, or the decision to submit the study results for publication.


