Kidney transplantation in the elderly
Article information
Abstract
Interest in kidney transplant studies in the elderly population is increasing as more research has been conducted on the immune system. With this review, we hope to encourage the need for research on kidney transplantation in the elderly.
INTRODUCTION
The proportion of older Korean adults defined as aged 65 years or older has doubled in 17 years from 7% (an aging society) in 2000 to 14% (an aged society) in 2017, and is expected to grow to 21% (a super-aged society) by 2026 [1]. Therefore, the characteristics of elderly patients (frailty, multiple diseases, multiple medications, cognitive impairment, and depression) must be considered during treatment [2,3].
The number of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) patients is increasing worldwide [4]. In particular, metabolic diseases, such as diabetes mellitus and hypertension, are showing increasing incidence, and the number of ESKD patients requiring dialysis is growing rapidly [4]. According to the Korean Renal Data System, the average age of a dialysis patient is 65 years, and patients older than 65 years account for half of dialysis patients [4].
Kidney transplantation (KT) provides a good prognosis for ESKD patients [5]. In addition, advanced age is no longer an absolute contraindication [5]. The prognosis of KT is better than that of dialysis even in elderly ESKD patients, and the transplant survival rate has also improved [5].
According to the 2019 Korean Network for Organ Sharing annual report, the time for elderly dialysis patients to receive KT was 1,737 days longer for those older than 75 years than for those younger than 75 years [6]. In other words, although KT for the elderly is not an absolute contraindication, it is not performed regularly. We aim to assess the changes in research on KT in the elderly and determine where more studies are needed in the future. We hope that KT will be encouraged for elderly patients through this article.
IMMUNE SYSTEM IN THE ELDERLY
Age-related changes in the immune system, known as immunosenescence, are associated with complex changes and dysregulation. Immunosenescence is thought to occur, in part, as a result of degeneration of the thymus [7]. The production of T and B cells gradually decreases compared to their demand, which results in increases in the number of effector/memory cells (Fig. 1) [7].
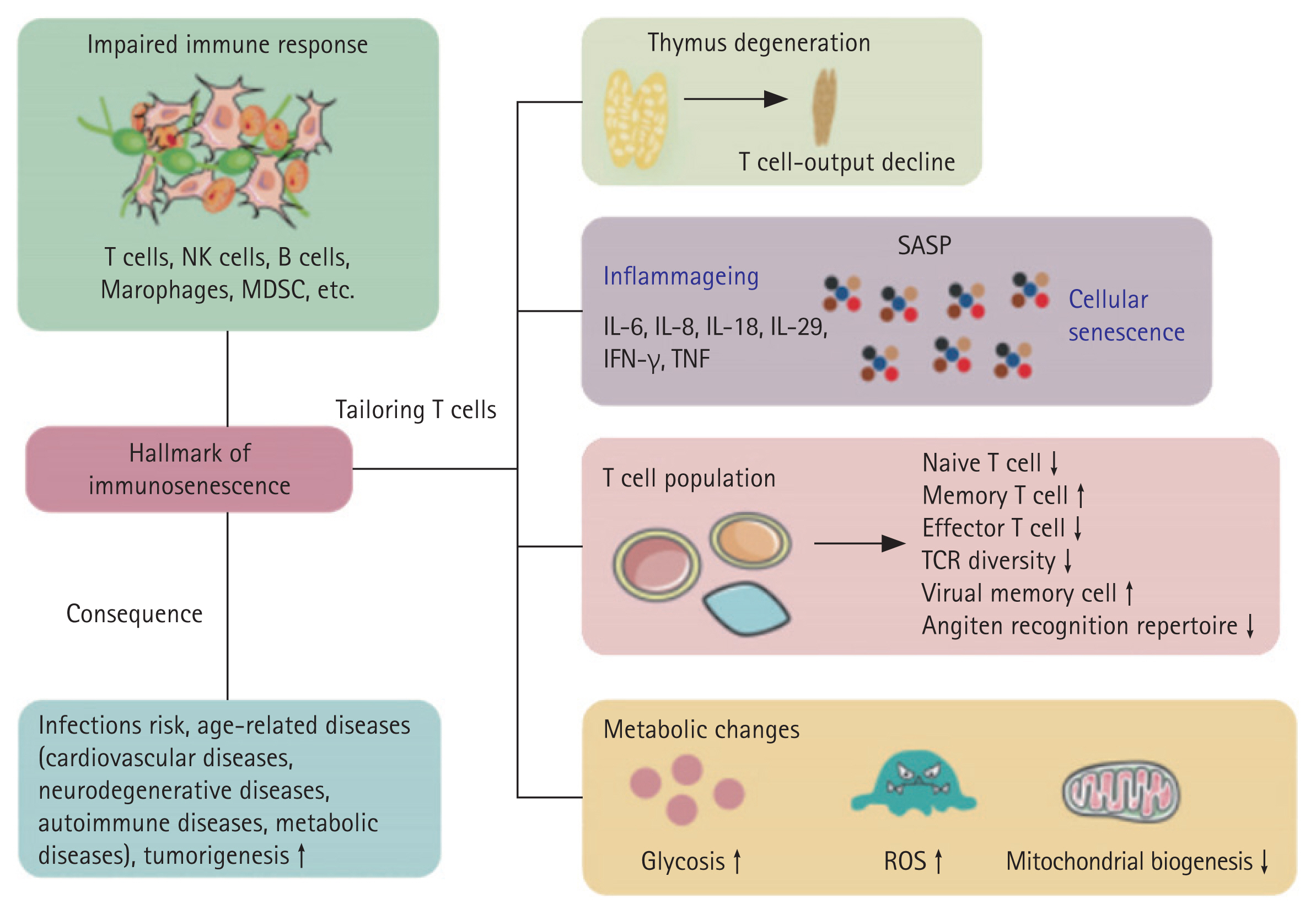
Hallmarks of immunosenescence and related diseases. IFN-γ, interferon-γ; IL, interleukin; MDSC, myeloid-derived suppressor cells; NK cells, natural killer cells; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; TCR, T-cell receptor; TNF, tumor necrosis factor. Adapted from Lin et al. Signal Transduct Target Ther 2023;8:200 [29].
The functions of neutrophils, NK cells, and monocytes/macrophages in the innate immune system become more difficult to control in old age [7]. This dysregulation is believed to contribute to increased susceptibility to infections, as well as increased symptoms associated with chronic inflammation and inflammatory diseases [7]. A decrease in myeloid cell production during aging leads to an increase in immature CD56bright NK cells and an increase in mature CD56dim NK cells, promoting an overall change in the distribution of NK cells [8]. Recent research has revealed that mortality in the elderly is associated with low NK cell activity [7]. Additionally, the low seroconversion that occurs after flu vaccination in the elderly indicates that NK cell activity is reduced [9]. However, whether the absolute number of NK cells changes with age is controversial [10].
A recent study suggested that macrophage polarization is more complex than previously thought and consists of a spectrum rather than two defined lineages [11]. Additionally, phagocytosis and intracellular tumor necrosis factor-α concentrations are higher in people aged 65 years or older after using a toll-like receptor 4 agonist than in younger people in general [12].
Dendritic cells (DCs) play an essential role in inducing and regulating T-cell responses through antigen presentation in response to stimuli [13]. Monocyte-derived DCs are impaired in the upregulation of interleukin (IL)-8 and monocyte chemoattractant protein-1 (MCP-1) and the secretion of IL-6 in older adults [14]. Recent results have shown that upregulation of IL-8 and MCP-1 and IL-6 secretion are impaired in monocyte-derived DCs from older adults and have a reduced ability to prime CD4+ T cells [14]. However, it has not been determined whether the inability to prime T cells is due to impaired antigen presentation or the response to antigen presentation [14].
Various subsets within the CD4+ T cell population are present in the adaptive immune system, including Th1, among which Th2, Th17, and regulatory T cells (Tregs) exhibit unique age-related characteristics [15,16]. A higher proportion of Th2 (CD3 + CCR4+) cells is present in aged individuals. The Th2/Th1 ratio of individuals with an average age of 91 years (referred to as the elderly population) is similar to that of younger adults [17]. These results suggest that maintaining an abundant Th1 population is important for healthy aging [17]. Older adults (≥ 65 yr) have an increased proportion of Th17 cells and an enhanced Th17 function, as well as a reduced Treg population, compared to middle-aged and younger adults [17]. Changes in these proportions ultimately result in a skew toward a high Th17 to Treg ratio in older adults, which may contribute to the imbalance between the pro- and anti-inflammatory responses observed in aging [18].
Recent studies have shown that the absolute number of circulating naïve CD4+ cells decreases as the thymus degenerates, although age-related changes in the thymus are not constant throughout life [19]. Additionally, due to internal and external factors, thymic involution and possible immunosenescence are not well described and require further investigation [19].
COMPLICATIONS OF KT IN THE ELDERLY
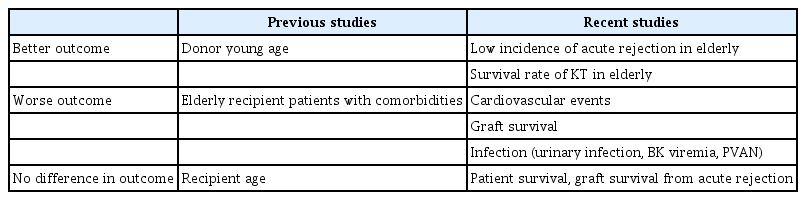
Previous studies have demonstrated that the prognosis of KT is better than that of dialysis in elderly patients with ESKD [20–22]. Recent studies have shown that the survival rate from KT in the elderly increases after considering their pharmacokinetic and immunological characteristics. A study published in 2009 on the immune system and pharmacokinetics of older adults [23] showed that absorption capacity, volume of distribution, hepatic metabolic function, and renal function decrease with age, resulting in changes in the disposition of drugs in the body [23].
Effect of age on KT
Donor age is an important factor affecting graft function and patient survival. The only independent predictor of graft survival and recovery of renal function after KT is donor age, and younger age is associated with better outcomes [24–27]. Subsequent studies have confirmed that donor age is an important factor affecting the prognosis and survival rate after KT. The younger the donor, the better the prognosis after transplantation [28].
Infectious complications
Elderly recipients are at a higher risk of developing infections, which are associated with an increased risk of complications [23]. Bacterial and viral infections are particular problems in the elderly population, particularly urinary infections, BK viremia, and polyomavirus-associated nephropathy [23,29].
A study conducted in 2007 suggested that allocating kidneys to elderly patients with comorbidities may not be effective and suggested further discussion of the exclusion criteria [30].
Elderly recipients aged 60 or older have significantly higher mortality rates, particularly infection-related mortality, but do not have an increased risk of graft failure or acute rejection [24]. Although there is a high rate of infection-related complications in elderly recipients, there is a low incidence of acute rejection [29]. This is likely because of the less active T and B cells in the immune systems of older adults [29].
In a study comparing graft survival by recipient age, the 1-year and 5-year overall patient survival rates were 90% and 76% for recipients older than 60 years, and the overall 1- and 5-year actuarial graft survival rates were 84% and 64%, respectively [25]. No significant differences were observed in graft survival or prognosis in recipients aged 60 or older compared with younger recipients [25].
Cardiovascular complications
Older transplant recipients have a higher risk of death from a cardiovascular event than younger recipients [31]. The leading causes of death are heart disease, infections, and malignancies. Death is seven times more likely if the graft works properly. Death due to a functioning graft was the most common cause [31].
Graft survival
Graft survival in elderly patients is worse than in younger patients [23,29]. There is a high risk of graft failure, particularly if the kidney is from an older donor [23,29]. The risk of acute rejection depends on the donor age because kidneys from older donors may have a lower regenerative capacity to tissue injury, increasing immunogenicity [23].
Graft survival and survival from acute rejection in elderly recipients have not been inferior to those of younger recipients over the past 2 to 3 years [29]. No difference was detected in the incidence of delayed graft function, graft failure, or acute rejection between groups older and younger than 60 years [29].
USE OF IMMUNOSUPPRESSANTS AFTER KT IN THE ELDERLY
The ability of the body to effectively utilize lipids is reduced in the elderly compared to younger people. Due to the aging of the metabolic mechanisms, the total body clearance rate of immunosuppressants decreases and the drug concentration in the body increases [23]. Thus, at a fixed drug concentration, the effect can be similar to that seen in younger people [23].
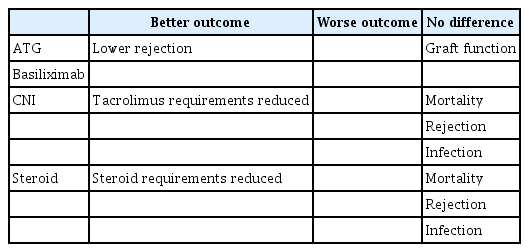
If a constant dose of 1.5 mg/kg per day is given to an elderly recipient for 4 days as antithymoglobulin (ATG) induction therapy, it can theoretically exist in the body at 0.75 mg/kg per day [32]. Although capacity could be adjusted in this way, further testing is required [32]. Another retrospective analysis of more than 300 elderly KT recipients (≥ 60 yr) treated with reduced cumulative ATG doses showed similar graft function but lower rejection rates compared to younger patients (< 60 yr) [33].
Other induction therapy studies have shown that lower immunosuppression intensity may help reduce mortality in elderly patients [34]. Tacrolimus and steroid requirements were lower in a study comparing basiliximab and ATG in elderly patients with low-risk KT, and no differences in all-cause mortality, rejection, or infection were observed [34].
When calcineurin inhibitors (CNIs) are administered to elderly patients as maintenance therapy, the total clearance in the body decreases by 34%, but the concentration in the body is 44% higher than in younger people [35]. Mortality, particularly infection-related death, increases in older recipients [32,35]. To control these complications, studies recommend age-appropriate immunosuppression in KT recipients [32,35]. For example, the CNI trough level is 6–8 ng/dL for the first 6 months after transplantation and 4–6 ng/dL thereafter. However, these therapies are optional recommendations, and clinical trials in elderly patients are rare [32,35].
Impaired liver function in elderly patients is associated with changes in plasma protein levels, which affects the binding of glucocorticoids to plasma proteins [36,37]. These pharmacokinetic characteristics indicate that a dose adjustment compared to younger patients is necessary when using glucocorticoids in elderly KT patients [36,37]. Nevertheless, clinical trials targeting the elderly are lacking [36,37].
TRACKING MARKERS AFTER KT IN THE ELDERLY
Limited data are available on adequate post-donation renal recovery for predicting remaining long-term kidney function [38]. This should be resolved in future studies with larger populations and longer follow-up periods [38]. Monitoring blood levels may not adequately reflect the effects of immune aging or age-related impaired organ function [39]. Diagnostic use of biomarkers in elderly transplant recipients may be an alternative [39].
Assessing calcineurin phosphatase activity and down-stream product nuclear factors of activated T cells has been used as a diagnostic tool to assess the intracellular effectiveness of CNI in older adults [40]. Additionally, inosine-monophosphate dehydrogenase has been used against the mTOR-dependent kinases p70S6 103 and MPA 104 to assess the effectiveness of the corresponding target enzymes [40].
Thymic function before transplantation is correlated with the rate of malignancy after transplantation [41]. Consequently, assessing rearranged ablation circles may help predict post-transplantation malignancy after ATG treatment [41].
Additionally, several soluble biomarkers detectable in blood and urine have been studied to monitor the functional immune responses after KT [42,43]. Studies measuring intracellular adenosine triphosphate production to assess cell-mediated immune responses have correlated it with rates of infection and cell rejection [44].
In a recent study, proteinuria 3–6 months post-transplant was a surrogate marker for predicting early renal outcomes in elderly KT patients [45]. The severity of the proteinuria after KT is inversely associated with graft survival and a favorable patient outcome [45]. This result has been used to predict rejection after KT in the elderly [45].
A high neutrophil-to-lymphocyte ratio (NLR) is associated with higher proteinuria, higher serum creatinine levels, lower estimated glomerular filtration rates, and a higher frequency of advanced CKD [46]. Consequently, a high NLR reflects a more advanced stage of CKD and suggests a role for NLR as a biomarker for predicting the progression of CKD [46]. Although this study was conducted on CKD patients, it is expected to be repeated in elderly KT patients [46].
CONCLUSION
Age broadly affects pharmacodynamics, pharmacokinetics, and immune responses. KT has many side effects in the elderly and requires precise guidelines for the use of immunosuppressants.
The number of CKD patients has been increasing, as has the number of elderly dialysis patients. Therefore, further research on KT in the elderly is needed, and precise standards for immunosuppressant medication and markers for post-transplant follow-up should be established.
Notes
CRedit authorship contributions
Byung Hwa Park: investigation, data curation, software, writing - original draft, writing - review & editing; Song Yi Kil: software, writing - review & editing, visualization; Ye Na Kim: visualization, supervision; Ho Sik Shin: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - review & editing, visualization, supervision; Yeonsoon Jung: conceptualization, formal analysis, visualization, supervision; Hark Rim: conceptualization, formal analysis, visualization, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None