Association between oral health and hyperuricemia in Korean adults: Korea National Health and Nutrition Examination Survey 2016–2019
Article information
Abstract
Background/Aims
Hyperuricemia plays an essential role in the gout. Despite the clinical significance of hyperuricemia, a direct relationship between oral health and hyperuricemia has not been established. We aim to investigate the association between oral health and hyperuricemia.
Methods
We selected 17,557 subjects from the Korea National Health and Nutrition Examination Survey database for the years 2016–2019. Oral health-related variables included the number of dental caries, regular tooth brushing, use of secondary oral products, and regular dental examinations. The odds ratio (OR) and 95% confidence intervals (CIs) for hyperuricemia were calculated using a multivariable-adjusted logistic regression model.
Results
Oral health status with dental caries and oral health behaviors, including tooth brushing, secondary oral products, and regular dental examination, were significantly associated with hyperuricemia in all participants. The adjusted OR and 95% CIs for hyperuricemia comparing more than three dental caries with no dental caries were 1.28 (1.08–1.52). The adjusted OR and 95% CIs for hyperuricemia in regular tooth brushing, use of secondary oral products, and regular dental examination were 0.78 (0.67–0.91), 0.91 (0.83–1.00), and 0.86 (0.78–0.95), respectively. Notably, the association between oral health and hyperuricemia was more prominent in male subjects. In addition, when subjects were grouped by the oral health scoring system, the prevalence of hyperuricemia was lower in groups with better oral health scores.
Conclusions
We demonstrated that oral health status and behaviors are associated with hyperuricemia, particularly in males. Further studies are necessary to confirm the association between oral health and hyperuricemia.
INTRODUCTION
Hyperuricemia is a metabolic disease in which uric acid levels remain excessively high. Hyperuricemia plays an essential role in the development of gout and is well-known as a prerequisite of gout. In addition, hyperuricemia has important clinical implications because it is considered a risk factor for coronary heart disease, hypertension, insulin resistance, stroke, and death [1–4]. Nevertheless, the prevalence rate of hyperuricemia is 11.4% in Korea and 12.7% in the United States [5,6] and the prevalence of gout is increasing rapidly in Korea [7].
The oral cavity is a complex environment with various microorganisms [8]. Unless appropriately managed, inflammatory conditions, such as dental caries and periodontitis, may occur. Furthermore, oral health is known to be related to pneumonia, cardiovascular diseases, and other oral diseases [9,10]. Oral health is critically affected by oral hygiene and can be improved through regular self-management. Therefore, it would be important to investigate specific diseases related to oral health and prevent them [11].
Despite the clinical importance of hyperuricemia, little is known about its prevention. Although several self-managed interventions can improve oral health, a direct relationship between oral health and hyperuricemia has not been established. Therefore, we aim to investigate the association between oral health and hyperuricemia in Korean adults using the nationwide population-based Korea National Health and Nutrition Examination Survey (KNHANES) database.
METHODS
Data source and study population
The KNHANES is a cross-sectional survey and a nationally representative database of the Korean population managed by the Korea Centers for Disease Control and Prevention (KCDC) [12]. The KNHANES database includes physical examinations, blood test results, and health-related interviews, including oral health behaviors. To conduct the KNHANES, trained staff interviewed subjects and applied standardized health examination protocols [13]. This study used data from the KNHANES 2016–2019 because this database contains blood test results for uric acid levels. Informed consent was obtained from all participants, and the KNHANES was approved by the Institutional Review Board of the KCDC. This study used anonymized KNHANES data, and the protocol was approved by the Institutional Review Board of Dong-A University Hospital (DAUHIRB-EXP-21-106).
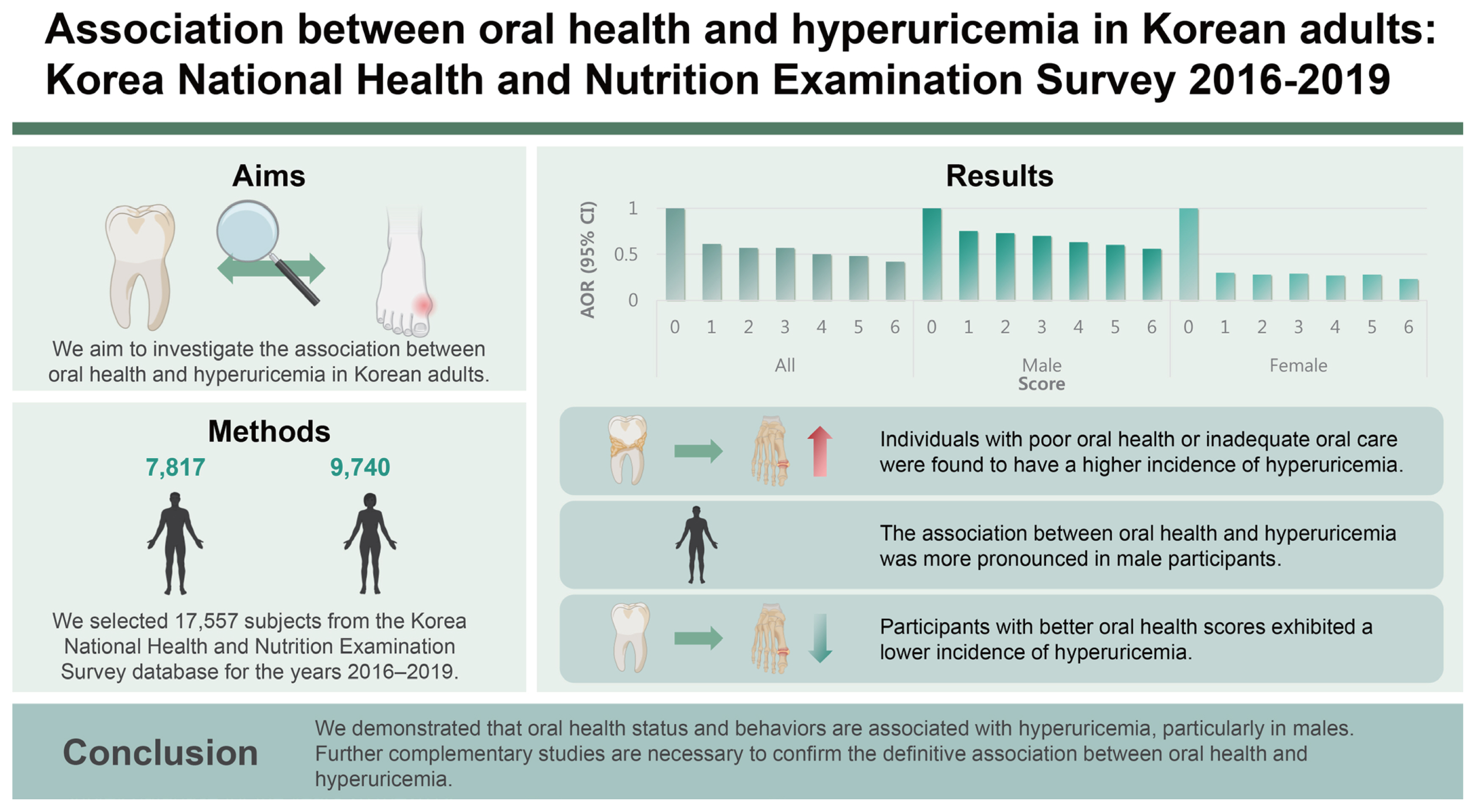
From 23,335 subjects in the KNHANES 2016–2019 database, we excluded 3,255 subjects without uric acid data. Subjects under the age of 20 (n = 2,063), pregnant (n = 69), or with missing values (n = 391) were also excluded. Finally, this study included 17,557 subjects (7,817 males and 9,740 females). Subsequently, we investigated the association between oral health and uric acid. A flow of the study population is shown in Figure 1.
Definition of oral health-related variables
The oral health examination program comprised oral examinations conducted by trained dentists and self-report questionnaires. The number of dental caries was categorized as 0, 1–2, or ≥ 3. Oral health-related questionnaires included questions on the time of day when subjects brushed their teeth and used secondary oral products, as well as the presence of regular dental examination as oral health behaviors. The time of day for tooth brushing was classified as before or following breakfast, lunch, and dinner or before bedtime and after snack. We calculated the frequency of daily tooth brushing. The number of tooth brushing was categorized as ≤1, 2, or ≥3. The secondary oral products included the following: dental floss, mouthwash, interdental brushes, electric toothbrushes, irrigation devices, tongue cleaners, end-tufted brushes, and special devices for dentures. The presence of regular dental examinations within a year was also evaluated.
To evaluate the association between oral health and hyperuricemia, we devised and calculated the oral health score by scoring the oral health-related variables available in the database and summing them together. A schematic diagram of the oral health score is presented in Supplementary Table 1. Unlike a few other scoring systems for the evaluation of oral health [14,15], the oral health score in this study comprises the numbers of dental caries and efforts to maintain oral hygiene. Therefore, we hypothesized that the oral health score in this study is related to oral hygiene. For example, A higher oral health score suggests better oral hygiene, and a lower oral health score indicates poor oral hygiene.
Clinical variable measurements and definitions
Body mass index (BMI) was calculated using the formula weight (kg) divided by height squared (m2). All subjects fasted for at least eight hours before blood sampling. Blood samples were processed and refrigerated immediately for transportation to the Central Testing Institute (NeoDin Medical Institute, Seoul, Korea). Uric acid, fasting glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), and creatinine were measured using a Hitachi Automatic Analyzer 7600-210 (Hitachi, Tokyo, Japan). The estimated glomerular filtration rate (GFR) was calculated using the modification of diet in renal disease equation [16]. Hyperuricemia was defined as a serum uric acid level ≥ 7.0 mg/dL in males and ≥ 6.0 mg/dL in females [17].
Hypertension status was categorized into three groups: (a) hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or antihypertensive medication use; (b) prehypertension was defined as 120 mmHg ≤ SBP < 140 mmHg or 80 mmHg ≤ DBP < 90 mmHg; and (c) normal hypertension status was defined as SBP < 120 mmHg and DBP < 80 mmHg [18]. Diabetes status was categorized into three groups: (a) diabetes was defined as fasting blood sugar (FBS) ≥ 126 mg/dL or taking anti-diabetic medication; (b) pre-diabetes was defined as 100 mg/dL ≤ FBS < 126 mg/dL; and (c) normal status was defined as FBS < 100 mg/dL [19]. Using the dyslipidemia criteria for Koreans, dyslipidemia status was categorized into two groups with cutoff values as follows: total cholesterol ≥ 240 mg/dL, triglycerides ≥ 200 mg/dL, and HDL-C ≤ 40 mg/dL [20]. Furthermore, we extracted information on cancer and depression status from the survey.
Income level was divided into four groups according to the standard income quartile of the sample population. The higher the income quartile is, the greater the income. Smoking status was categorized into current smokers, ex-smokers, and non-smokers. Alcohol consumption status was categorized into two groups: (a) non-consumers, with no alcohol consumption in the past year or less than once a month, and (b) consumers, with alcohol consumption more than once a month. Physical exercise status was categorized into two groups: (a) the regular exercise group, in which participants performed moderately hard exercise for at least 150 minutes a week, hard exercise for at least 75 minutes a week, or mixed exercise equivalent to the above level (1 minute of hard exercise equivalent to 2 minutes of moderately hard exercise); and (b) the non-regular exercise group, in which participants performed physical activity less than the above-mentioned level.
Statistical analysis
All statistical analyses were performed using a complex sample design in SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA) and R 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). The characteristics of the subjects were analyzed according to their uric acid levels. Continuous variables are presented as the means and standard deviations, and categorical variables are presented as the number of cases with percentages. Continuous variables are presented as the means with standard errors, and categorical variables are presented as the percentages with standard errors. Logistic regression analysis was performed, and odds ratios (ORs) with 95% confidence intervals (CIs) for hyperuricemia were calculated to investigate the association between oral health and hyperuricemia. The multivariable-adjusted logistic regression analysis was adjusted for age, sex, income level, BMI, GFR, hypertension status, diabetes status, dyslipidemia status, cancer status, depression status, smoking status, alcohol consumption status, and regular exercise status. A p value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study population
The baseline characteristics of the subjects according to their uric acid levels are presented in Table 1. There were significant differences between the groups with no hyperuricemia and those with hyperuricemia, except for the exercise variable. The mean uric acid levels in each group were 4.8 and 7.5 mg/dL, respectively. The hyperuricemia group included young males. In addition, compared to the no hyperuricemia group, the hyperuricemia group had higher BMI, SBP, DBP, total cholesterol, and triglyceride levels but lower HDL-C and GFR levels. Comorbidities, including hypertension, diabetes, and dyslipidemia, were more common in the hyperuricemia group. A greater number of dental caries and a lower number of tooth brushing were more common in the hyperuricemia group. The use of secondary oral products and regular dental examinations were more frequent in the no hyperuricemia group. The baseline characteristics of the subjects divided into male and female are presented in Supplementary Table 2 and 3.
Association between oral health and hyperuricemia
The associations between oral health and hyperuricemia are presented in Table 2. The multivariable-adjusted logistic regression analyses were performed. Oral health status with dental caries and oral health behaviors, including tooth brushing, the use of secondary oral products, and regular dental examination, were significantly associated with hyperuricemia in all the participants. The adjusted OR and 95% CIs for hyperuricemia comparing more than three dental caries with no dental caries were 1.28 (1.08–1.52). The adjusted OR and 95% CIs for hyperuricemia comparing more than three tooth brushing with under one tooth brushing were 0.78 (0.67–0.91). The adjusted ORs and 95% CIs for hyperuricemia associated with secondary oral products and regular dental examinations were 0.91 (0.83–1.00) and 0.86 (0.78–0.95), respectively. When the study was expanded to include sex stratification, the male group exhibited a significant association with oral health and hyperuricemia. In addition, subgroup analysis according to pre- and post-menopausal women can be found in Supplementary Table 4. There was no consistent correlation between oral health and hyperuricemia depending on pre- and post-menopausal women.
Association between the oral health score and hyperuricemia
We calculated the oral health score using oral health status and behaviors. The association between the oral health score and hyperuricemia incidence is presented in Figure 2. There was a significant tendency between the oral health score and hyperuricemia regardless of the study population (p for trend < 0.05). Specifically, the adjusted OR and 95% CIs for hyperuricemia comparing score 6 with score 0 were 0.42 (0.27–0.64) for all subjects, 0.56 (0.34–0.94) for male subjects, and 0.23 (0.10–0.50) for female subjects. The subgroup analysis between pre- and post-menopausal women for the association between the oral health score and hyperuricemia incidence is described in Supplementary Table 5. When the female population was divided into pre- and post-menopausal states, the adjusted OR and 95% CIs for hyperuricemia comparing score 6 with score 0 were 0.20 (0.05–0.81) for premenopausal women and 0.28 (0.11–0.76) for postmenopausal women, respectively. In addition, the subgroup analysis based on age is described in Supplementary Table 6. When the study population was divided by age (over 65 yr), the adjusted OR and 95% CI for hyperuricemia comparing score 6 with score 0 were 0.55 (0.31–0.98) for those under 65 years of age and 0.40 (0.18–0.86) for those above 65 years of age.
DISCUSSION
This study investigated the association between oral hygiene and hyperuricemia using a large-scale, cross-sectional study with KNHANES data. The number of dental caries was associated with hyperuricemia, and oral hygiene behaviors, such as regular tooth brushing, the use of secondary oral products, and regular dental examination, were also related to hyperuricemia in all subjects. Furthermore, we applied the oral health score by grading oral health-related variables and summing them together. As a result, a significant association between the oral health score and hyperuricemia was also confirmed. According to the sex-based subgroup analysis, a higher oral health score was associated with a lower incidence of hyperuricemia regardless of sex. In detail, a statistically significant relationship between higher oral health scores and a lower incidence of hyperuricemia was observed in the male subgroup. However, in the female group, only a small difference between oral health score groups 1~6 was observed, and the group with an oral health score of 0had a remarkably greater incidence of hyperuricemia. There were no significant differences in trend between the premenopausal and postmenopausal subgroup analyses. According to the age-based subgroup analysis, a similar correlation between the oral health score and hyperuricemia was maintained in both groups, but this correlation was statistically valid only in the elderly group (aged 65 to 80 yr).
Although several previous studies exist, the association between oral disease and hyperuricemia has remained controversial. Recently, Byun et al. [21] analyzed the relationship between periodontitis and hyperuricemia in a large population and reported that the two diseases were not significantly correlated. On the other hand, Banu et al. [22] reported that blood uric acid levels in patients with periodontitis were greater than those in normal subjects. The former study may have had data reliability issues because it used data acquired over an extended period (from 2004 to 2016). In the latter study, there may have been problems with including a limited number of patients as samples. In this study, we used data from the KNHANES 2016–2019 database with the same protocol within a relatively short period. We secured a sufficient number of subjects for statistical analysis, which could be a strength of this study.
Although a clear mechanism underlying the relationship between oral hygiene and hyperuricemia is unknown, several perspectives can be derived from previous studies. First, the change in the oral microbiota caused by poor oral hygiene can be mentioned, as the oral microbiota is known for its association with various diseases [23]. In a previous study, Prevotella intermedia, which is known as one of the causative bacteria of tooth decay and periodontitis, was found to be abundant in the oral microbiota of gout patients [24]. In addition, the color of the root of the tongue, which may be related to oral health and oral microbiota, is indicative of hyperuricemia [25]. Furthermore, it has been reported that abnormal findings of the oral microbiota are also associated with severe asthma, cardiovascular disease, diabetes, and rheumatoid arthritis [26–32]. Most importantly, Sheng et al. [33] recently reported that hyperuricemia is associated with changes in the gut microbiota. As the composition of the oral microbiota is known to affect the composition of the gut microbiota [34], poor oral hygiene may cause changes in the oral microbiota, which may explain the association between oral hygiene and hyperuricemia [35]. Second, oral disease itself is known to be associated with metabolic disorders. Consistent with the results of this study, a previous meta-analysis study also revealed an association between periodontitis and hypertension [36]. In addition, Kobayashi et al. [37] reported that oral hygiene is associated with metabolic syndrome. According to a recent randomized controlled trial, the risk of metabolic syndrome was lower in the group with oral hygiene intervention than in the group without intervention [38]. Taken together, these findings suggest that oral hygiene may be related to hyperuricemia and metabolic disorders.
This study has several limitations. First, the oral health-related variables did not include the presence of periodontitis, a common clinical manifestation of an individual’s oral hygiene. However, in patients with periodontitis, dental examinations and radiological examinations are necessary. Furthermore, since the severity of periodontitis may vary from patient to patient, we quantitatively evaluated the patient’s oral health status by counting the number of dental caries. Second, oral health status and behaviors were investigated using a self-report questionnaire. Third, there is a possibility that unadjusted covariates were not analyzed in this study. Fourth, as our categories of the oral health score comprise efforts to prevent oral diseases, there is a possibility that individuals in the low oral health score group have lower concerns about their health status, resulting in hyperuricemia. Finally, this cross-sectional study aimed to determine the association between oral hygiene and hyperuricemia but could not establish a cause-and-effect relationship. We expect that a future study will be able to identify the predecessor relationship between oral hygiene and hyperuricemia.
In conclusion, we demonstrated that oral health status and behaviors are associated with hyperuricemia, especially in males. In addition, when subjects are grouped by the oral health scoring system, the prevalence of hyperuricemia is lower in groups with better oral health scores. Further complementary studies are needed to confirm a definitive association between oral health and hyperuricemia.
KEY MESSAGE
1. We demonstrated that oral health status and behaviors are associated with hyperuricemia, especially in males.
2. When subjects are grouped by the oral health scoring system, the prevalence of hyperuricemia is lower in groups with better oral health scores.
Notes
CRedit authorship contributions
Junyong Park: validation, writing - original draft, project administration, funding acquisition; Minkook Son: resources, investigation, formal analysis, visualization; Sung Won Lee: resources, validation, writing - review & editing, supervision; Won Tae Chung: software, writing - review & editing, visualization, supervision; Sang Yeob Lee: conceptualization, writing - review & editing, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by the Dong-A University research fund.
Data availability
The data are available from the Korea National Health and Nutrition Examination Survey (KNHANES), which was performed by the Korea Centers for Disease Control and Prevention (KCDCP), and are freely available from the website (https://knhanes.kdca.go.kr/knhanes/eng/index.do).