Prevalence and risk factors for gallstone and renal stone formation in patients with intestinal Behçet’s disease
Article information
Abstract
Background/Aims
The association between inflammatory bowel disease (IBD) and gallstone and renal stone formation has been established. However, few studies have investigated this association in patients with intestinal Behçet’s disease (BD). We aimed to examine the prevalence of gallstones and renal stones in patients with intestinal BD and identify potential risk factors.
Methods
We analyzed gallstone and renal stone occurrences in 553 patients diagnosed with intestinal BD who had undergone cross-sectional imaging examinations between March 2005 and April 2021 at the IBD Center, Severance Hospital, Seoul, South Korea. Logistic regression models were used to identify risk factors for gallstone and renal stone formation.
Results
Of 553 patients over a mean 12.1-year duration, 141 (25.4%) patients had gallstones and 35 (6.3%) had renal stones. In multivariate logistic regression analysis, disease duration > 19 years (OR 2.91, 95% CI 1.56–5.44, p < 0.001), prior intestinal BD-related surgery (OR 2.29, 95% CI 1.42–3.68, p < 0.001), and disease activity index for intestinal BD scores ≥ 75 (OR 2.23, 95% CI 1.12–4.45, p = 0.022) were associated with increased gallstone occurrence. A positive correlation was observed between renal stones, disease duration > 19 years (OR 5.61, 95% CI 1.98–15.90, p = 0.001) and frequent hospitalization (> 3 times) (OR 3.29, 95% CI 1.52–7.13, p = 0.002). No significant correlation was observed between gallstone and renal stone occurrence.
Conclusions
These findings contribute to greater understanding concerning gallstone and renal stone prevalence and associated risk factors in patients with intestinal BD.
INTRODUCTION
Behçet’s disease (BD), a chronic recurrent inflammatory disorder affecting multiple systems, is characterized with recurrent oral and genital ulcerations, ocular lesions, skin manifestations, arthritis, vasculitis, and involvement of the gastrointestinal tract [1,2]. Intestinal BD occurs in 3–60% of patients with systemic BD [1,3,4]. Intestinal BD and inflammatory bowel disease (IBD) share similarities in terms of clinical manifestations related to various gastrointestinal symptoms and endoscopic findings, along with a close association between mucosal healing and a favorable clinical course [3,5]. However, unlike IBD, intestinal BD typically shows rare anorectal involvement and presents with distinct endoscopic features such as a fewer number of lesions, larger and deeper ulcerations, and round or oval-shaped ulcers with discrete and elevated borders [3,6]. Currently, the treatment approach for intestinal BD is similar to that of IBD [4,7–9].
The relationship between IBD, including ulcerative colitis (UC) and Crohn’s disease (CD), and gallstone and renal stone formation has been widely recognized [10–12]. In the general population, gallstones have been reported to be associated with risk factors such as obesity, physical inactivity, insulin resistance and diabetes mellitus, nonalcoholic fatty liver disease, high-calorie intake, prolonged fasting, liver cirrhosis, and the use of drugs such as fibrates and calcineurin inhibitors [13]. Patients with IBD, especially those who have undergone ileal resection or have terminal ileum involvement, are at a higher risk of gallstone formation. This increased risk has been attributed to bile acid malabsorption, elevated bilirubin levels in bile, and reduced gallbladder motility [14–17].
Regarding renal stone formation, weight gain, a high body mass index (BMI), diabetes mellitus, and systemic diseases such as primary hyperparathyroidism and renal tubular acidosis are suggested risk factors in the general population [18]. The main factors known to increase the occurrence of renal stones in patients with IBD are low urine volume, low urine pH, hyperoxaluria, and lower urinary concentrations of magnesium and citrate, which are associated with gastrointestinal manifestations of IBD such as diarrhea and malabsorption and also significant water loss from surgical procedures such as ileal resection and ileostomy [12,19,20].
There is currently a lack of research exploring the prevalence of gallstones and renal stones in patients with intestinal BD. Therefore, this study aimed to investigate the prevalence and identify risk factors for gallstone and renal stone formation in patients with intestinal BD.
METHODS
Patients
Data were collected from patients who had been diagnosed with intestinal BD at the IBD Center, Severance Hospital, Seoul, South Korea. Patients with intestinal BD were defined as those who met the diagnostic criteria based on colonoscopic findings and extraintestinal systemic manifestations. Patients were classified into definite, probable, and suspected groups according to the diagnostic criteria. Definite intestinal BD was characterized by typical intestinal ulcers with extraintestinal symptoms. Probable intestinal BD included typical intestinal ulcers with typical oral ulcers or atypical intestinal ulcers and extraintestinal symptoms. Suspected intestinal BD comprised typical intestinal ulcers without any extraintestinal symptoms or atypical intestinal ulcers with only typical oral ulcers [21]. The confirmation of gallstones or kidney stones typically relies on conducting cross-sectional imaging examinations such as abdominal-pelvic computed tomography (APCT) scans and/or abdominal ultrasonography, or by considering patients who have undergone cholecystectomy due to the presence of gallstones. Exclusion criteria comprised patients lost to follow-up with no available electronic medical records, those who did not undergo cross-sectional imaging examinations, and those whose diagnosis changed from intestinal BD to CD or UC. Initially, 776 patients were enrolled in our intestinal BD cohort. Among them, the diagnosis was changed for 48, essential imaging examinations were lacking for 171, and medical records were incomplete for 4. In total, data concerning 553 patients were collected from March 2005 to April 2021, and a retrospective review was conducted. Patients were divided into two groups based on the presence or absence of gallstones and kidney stones, which had been confirmed through imaging examinations such as APCT scans and abdominal ultrasonography. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University (IRB No: 4-2020-0686).
Measurements and outcomes
The demographic characteristics included patient sex, age, extraintestinal manifestations (EIMs), BMI, history of intestinal BD-related surgery, the disease activity index for intestinal Behçet’s disease (DAIBD) score at the time of diagnosis, medication history (5-aminosalicylic acids [5-ASAs], corticosteroids, immunomodulators, biologics, non-steroidal anti-inflammatory drugs [NSAIDs]), hypertension, diabetes mellitus, and hospitalization frequency. The DAIBD score is calculated based on the criteria used for diagnosing BD, which includes factors such as general well-being, fever, EIM, abdominal pain, abdominal mass, abdominal tenderness, intestinal complications, and the number of liquid stools.
Statistical analysis
Means and standard deviations (SDs) or medians and interquartile ranges (IQRs) were calculated for continuous variables, and numbers and percentages were calculated for categorical variables. The distribution of categorical variables was compared between the groups with gallstones/ renal stones and without gallstones/renal stones using a chi-squared test. We analyzed the risk factors for gallstone and renal stone formation in patients with intestinal BD. Univariate and multivariate analysis was performed using a logistic regression model to identify independent risk factors for developing gallstones and renal stones. A p value < 0.05 was considered statistically significant. Analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) software.
RESULTS
Demographic and clinical characteristics of patients with intestinal BD
This study comprised 553 patients (mean follow-up duration, 12.1 ± 7.4 yr; median, 11 yr; IQR, 6–16 yr). The study population included 305 (55.2%) females (mean age at diagnosis, 54.3 ± 11.1 yr; median, 55 yr; IQR, 45–64 yr). In total, 431 (77.9%) patients exhibited EIMs, and the mean BMI was 22.2 ± 2.5 kg/m2 (median, 22.1 kg/m2; IQR, 20.0–24.1 kg/m2). The patients were classified according to the diagnostic algorithm for intestinal BD; as a result, 149 were categorized as definite, 294 as probable, and 110 as suspected. A history of hypertension and diabetes mellitus at diagnosis of intestinal BD was observed in 7.6% and 5.8% of patients, respectively. A total of 167 (30.2%) patients had a history of previous intestinal BD-related surgery. Among them, 74 underwent right hemicolectomy, 73 underwent ileocecectomy, 7 underwent small-bowel resection, and 13 underwent other types of surgery (Supplementary Table 1). Regarding the DAIBD score at diagnosis of intestinal BD, 196 (35.4%) patients scored < 19, 153 (27.6%) scored between 20 and 39, 148 (26.7%) scored between 40 and 74, and 56 (10.1%) scored ≥ 75. Regarding previous medications on cross-sectional imaging examination, 528 (95.5%) patients received 5-ASA treatment, 212 (38.3%) received steroid treatment, 197 (35.6%) received immunomodulator treatment, 91 (16.5%) received biologics treatment, and 162 (29.3%) patients received NSAIDS. In total, 119 (21.5%) patients had been hospitalized ≥ 3 times (Table 1, 2). Regarding imaging examinations, APCT was conducted in 396 patients (71.6%), ultrasonography in 32 patients (5.7%), and both APCT and ultrasonography in 125 patients (22.6%) (Supplementary Table 2). Sample endoscopy and abdominal images are shown in Figures 1–4.

Colonoscopy and APCT images of a 55-year-old woman diagnosed with intestinal BD. The patient had skin lesions consistent with the extraintestinal manifestations of BD. There is a typical oval-shaped ulcer in the ileocecal valve and cecum adjacent to the ileocecal valve. (A) APCT findings reveal a small-sized radio-opaque gallstone (B, indicated by the red arrow). APCT, abdominal-pelvic computed tomography; BD, Behçet’s disease.
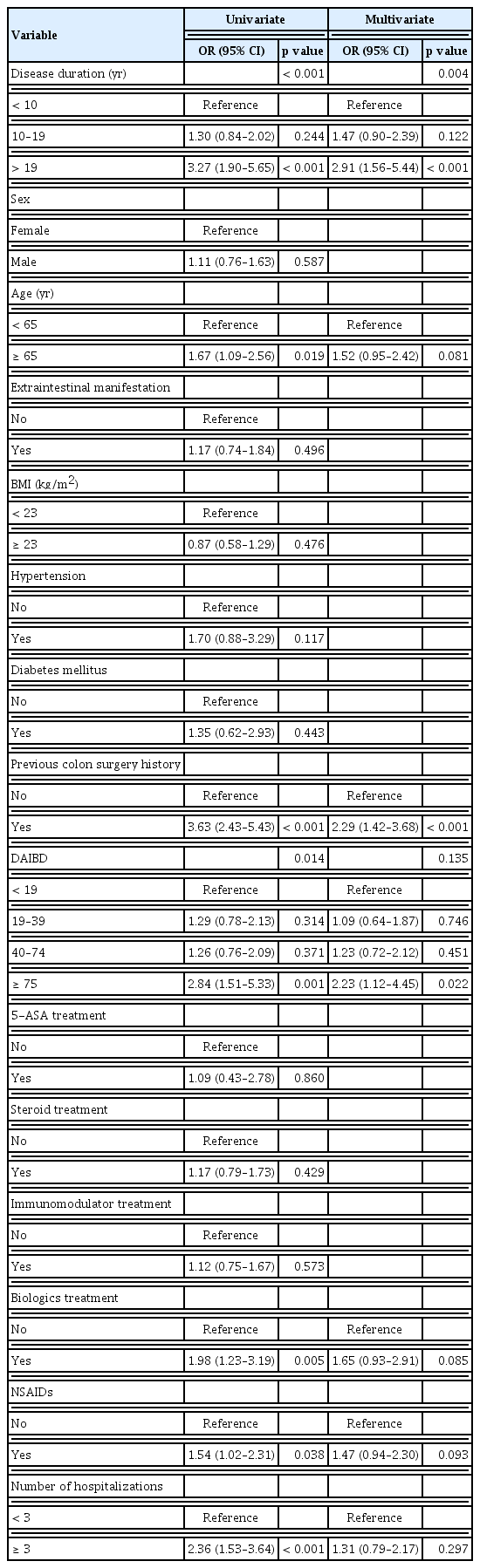
The images show endoscopic findings and the abdominal ultrasonography image of a 66-year-old woman with intestinal BD. The patient exhibited typical oral ulcers associated with BD. Several ulcers are visible in the ileocecal valve, along with edematous mucosal changes in the terminal ileum. (A) The ultrasonography image shows a 15-mm gallstone (B, indicated by red arrows). BD, Behçet’s disease.

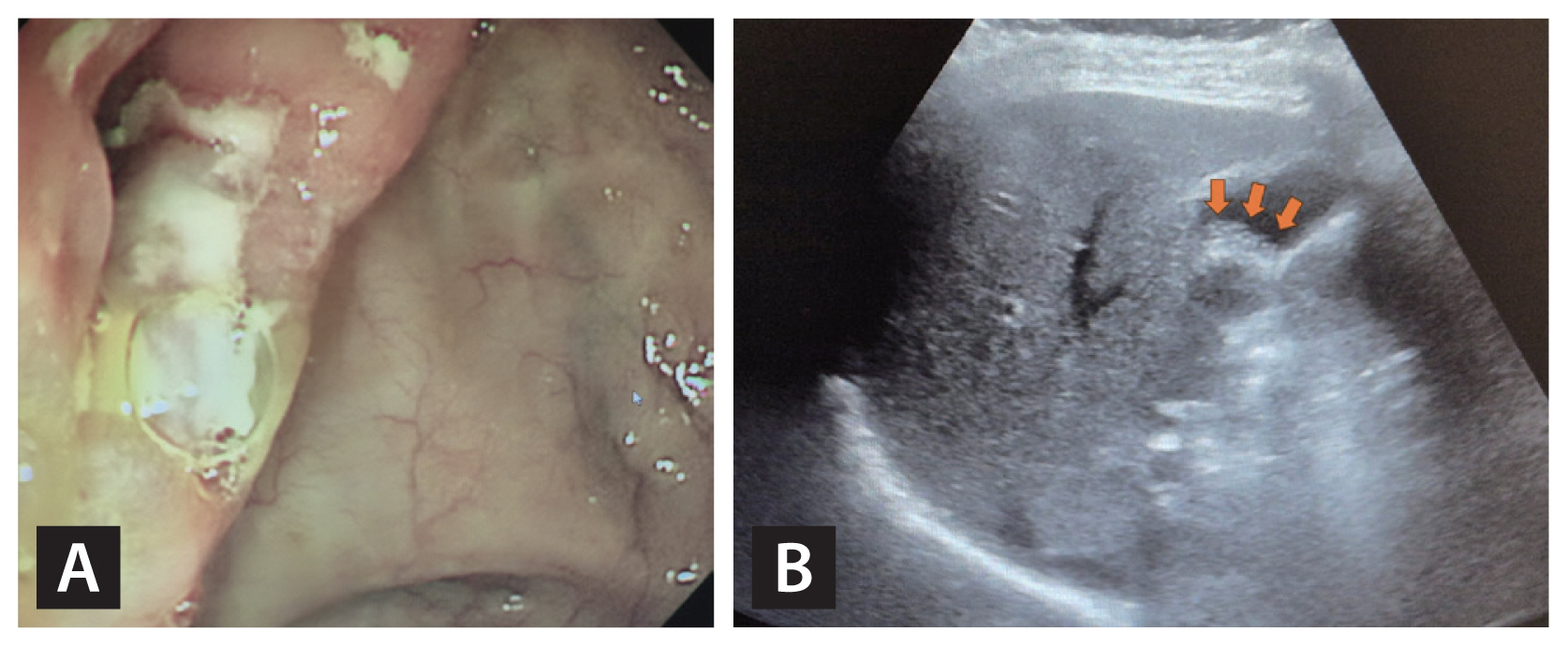
The patient exhibited typical oral ulcers and intestinal ulcer lesions consistent with intestinal BD. The endoscopic image shows edematous mucosa and ulcer lesions, leading to narrowing of the ileocecal valve. (A) The APCT image reveals a small stone in the right kidney (B, indicated by the red arrow). BD, Behçet’s disease; APCT, abdominal-pelvic computed tomography.
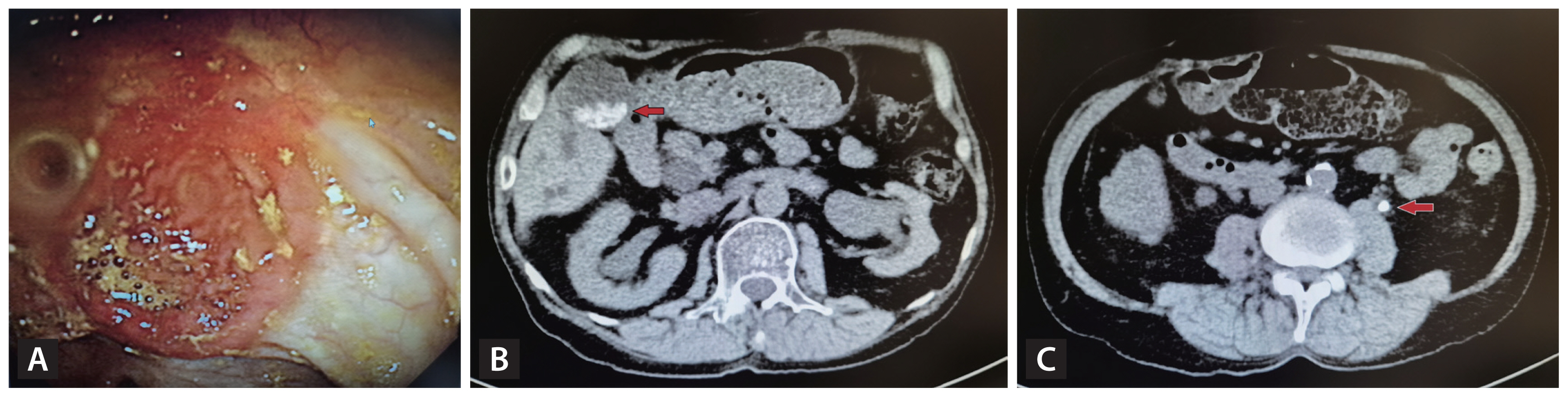
The patient underwent small-bowel resection due to complications associated with intestinal BD. The endoscopic image reveals an ulcerative lesion with edema and redness. (A) In the APCT image, sludges and stones are visible in the gallbladder (B, indicated by the red arrow) in addition to a 5-mm stone in the left ureter (C, indicated by the red arrow). BD, Behçet’s disease; APCT, abdominal-pelvic computed tomography.
Risk factors for gallstone formation in patients with intestinal BD
Of 553 patients, 141 (25.4%) had gallstones. In the univariate analysis, a disease duration > 19 years (odds ratio [OR] 3.27, 95% confidence interval [CI] 1.90–5.65; p < 0.001), patients aged ≥ 65 years (OR 1.67, 95% CI 1.09–2.56; p = 0.019), previous intestinal BD-related surgery history (OR 3.63, 95% CI 2.43–5.43; p < 0.001), and a DAIBD score ≥ 75 (OR 2.84, 95% CI 1.51–5.33; p = 0.001) showed a statistically significant association with gallstone occurrence. Both biologics treatment (OR 1.98, 95% CI 1.23–3.19; p = 0.005) and NSAID intake (OR 1.54, 95% CI 1.02–2.31; p = 0.038) showed statistically significant associations with gallstone occurrence. In the univariate analysis, a statistically significant association was observed between the number of hospitalizations (≥3 times) and gallstone occurrence (OR 2.36, 95% CI 1.53–3.64; p < 0.001).
In the multivariate logistic regression analysis, a disease duration >19 years (OR 2.91, 95% CI 1.56–5.44, p < 0.001), a previous colon surgery history (OR: 2.29, 95% CI 1.42– 3.68; p < 0.001), and a DAIBD score ≥ 75 (OR 2.23, 95% CI 1.12–4.45; p = 0.022) were positively associated with gallstone occurrence (Table 3).
Risk factors for renal stone formation in patients with intestinal BD
Of 553 patients with intestinal BD, 35 (6.3%) had renal stones. In the univariate analysis, a disease duration > 19 years (OR 6.56, 95% CI 2.40–17.90; p < 0.001), a history of previous intestinal BD-related surgery (OR 2.62, 95% CI 1.32–5.23; p = 0.006), and hospitalization ≥ 3 times (OR 3.85, 95% CI 1.92–7.74; p < 0.001) were found to be statistically significant.
In the multivariate analysis, disease duration > 19 years (OR 5.61, 95% CI 1.98–15.90; p = 0.001) and hospitalization ≥ 3 times (OR 3.29, 95% CI 1.52–7.13; p = 0.002) were positively associated with renal stone occurrence (Table 4).
The association between gallstones and renal stones
No significant association was observed between the prevalence of gallstones and renal stones. The presence of either gallstones or kidney stones did not increase the risk of developing one or other condition (OR 1.80, 95% CI 0.88–3.67; p = 0.106 and OR 1.80, 95% CI 0.88–3.67; p = 0.107, respectively).
DISCUSSION
Gallstone and renal stone diseases are highly prevalent conditions that impose significant challenges on healthcare systems worldwide. These conditions place a substantial burden on both patients and healthcare providers. The prevalence of gallstones ranges from approximately 10 to 15% among people of European ethnicity, whereas it tends to be lower in those of Asian and African ethnicities. In Asia, the prevalence of gallstones ranges from 3 to 10%, and from 2 to 5% in Korea specifically [22,23]. The annual prevalence of renal stones is approximately 3–5% globally, and the lifetime prevalence is estimated to be approximately 15–25% [24]. Several studies have previously reported the incidence and risk of gallstone and renal stone formation in patients with IBD. In one study, the incidence rates for gallstones were 14.35/1,000 persons/year in patients with CD and 7.48/1,000 persons/year in patients with UC [25]. Individuals with CD have a higher risk of developing gallstones [12,25–27]. In contrast to CD, the reported prevalence rates for gallstones in patients with UC remain controversial [25,28,29]. In our study, we found that the prevalence of gallstones in patients with intestinal BD was 25.4%. This finding is relatively high compared with UC and is similar or even higher compared with findings in relation to CD.
Compared with the general population, patients with IBD have a higher prevalence of renal stones. The prevalence of renal stones is reported to be between 0.2 and 11.0% among patients with non-colectomy UC, and ranges from 8.4 to 40.0% among patients with UC who have undergone bowel surgery [30–34]. In patients with CD, the reported frequency of renal stones ranges from 4.0 to 5.5% in those without bowel surgery and from 15.0 to 30.5% in those with bowel surgery [32,34]. In this study, the prevalence of renal stones in patients with intestinal BD was 6.32%, which is comparable to the prevalence rate for patients with CD.
We identified significant risk factors contributing to the occurrence of gallstones in patients with intestinal BD, such as a disease duration > 19 years, a history of prior intestinal BD-related surgery, and elevated disease activity (DAIBD score, ≥ 75) at the time of diagnosis. These trends show some consistency with the identified risk factors in patients with IBD, with a more pronounced pattern observed in CD compared to UC [25,26,35]. In patients with IBD, it has been hypothesized that a decrease in bile acid absorption may lead to increased cholesterol concentration in the bile, thereby raising the likelihood of gallstone formation. Additionally, during the course of IBD treatment including surgery, prolonged fasting or parenteral nutrition can adversely affect gallbladder motility, potentially increasing the risk of gallstone development. As the disease persists for a longer duration and becomes more severe in its activity, the risk of these events occurring becomes higher [12]. In patients with intestinal BD, similar to CD, ileocecal area involvement is common, leading to bile acid malabsorption and bile supersaturation. As the disease activity intensifies, there is a higher likelihood of requiring fasting periods or surgical interventions. Consequently, disease duration, surgical history, and disease activity appear to act as independent factors contributing to the increased occurrence of gallstones in intestinal BD, similar to that of IBD, and particularly that of CD.
Nephrolithiasis is due to the presence of an excessive concentration of components such as oxalate or urate. Low urine volume, low urine pH, low urine citrate or magnesium concentration, and high urine oxalate levels are pathological factors contributing to the development of renal stones [20]. In patients with IBD, the main factors contributing to the frequent occurrence of renal stones include a loss of water and salt in individuals with ileostomy or severe diarrhea. This leads to concentrated urine with reduced excretion of citrate and magnesium, which in turn promotes the accumulation of oxalate stones [36]. Additionally, abnormalities in intestinal absorption result in decreased calcium excretion in the feces, leading to an increase in calcium oxalate excretion in the urine [19]. Moreover, patients with ileostomy may experience increased alkaline fluid secretion, leading to acidic urine, which is also a factor in the development of renal stones [37]. Several studies have identified risk factors for a high prevalence of renal stones in patients with IBD. These factors include low serum bicarbonate levels, the presence of EIMs in IBD, the ileocolonic L3 location (CD), high disease activity (UC), a history of intestinal surgery, lack of physical activity, and the use of NSAIDs [12,38,39]. We found that a disease duration > 19 years and a history of ≥ 3 hospitalizations were statistically significant risk factors for the prevalence of renal stones in patients with intestinal BD. With prolonged disease duration or an increased number of hospitalizations, there is a higher likelihood of exposure to fluid loss, electrolyte imbalance, and dehydration, which could align with the pathophysiological processes contributing to renal stone formation.
Stone diseases are usually diagnosed using imaging examinations, such as APCT and ultrasonography. For diagnosing gallstones, ultrasonography reportedly has a sensitivity of 84% and specificity of 99% [40], while APCT has a sensitivity of 80% [41]. For diagnosing renal stones, ultrasonography reportedly has a sensitivity of 84% and specificity of 53% [42], whereas APCT has a sensitivity of 95% and specificity of 98% [43]. In this study, APCT was performed in most cases, while ultrasonography alone was used in only a handful of patients diagnosed with gallstones and renal stones.
This study is the first to analyze the prevalence and risk factors for gallstone and renal stone formation in patients with intestinal BD. Additionally, the study analyzed data from a substantial cohort of patients at a major tertiary hospital.
Our study had some limitations. First, no pre-diagnostic investigation was undertaken to exclude the occurrence of gallstones and renal stones prior to the diagnosis of intestinal BD; therefore, there is a possibility of bias in relation to these conditions. Second, as one of the largest centers, our institution mainly treats patients with more severe conditions who may have had a higher incidence of complications. This could potentially lead to an overestimation of the prevalence rate of stone diseases. Third, the absence of a control group comprising individuals without intestinal BD limits the ability to determine the precise risk of gallstones and renal stones that can be attributed to intestinal BD. Fourth, no specific evaluation was conducted to differentiate whether gallstones and renal stones were considered sufficiently mild to be managed through observation alone or if clinical intervention was necessary due to their clinical significance.
In conclusion, we conducted a long-term investigation of the prevalence of gallstones and renal stones in a large cohort of patients with intestinal BD. The identified risk factors for gallstone development were disease duration > 19 years, prior intestinal BD-related surgery, and elevated disease activity. Concerning renal stones, the risk factors were disease duration > 19 years and a history of more than three hospitalizations. Future research is required to investigate the prevalence of gallstones and renal stones in patients with intestinal BD compared with the general population.
KEY MESSAGE
1. When comparing it to the general population, it is already documented that patients with IBD like UC or CD have an increased susceptibility to conditions such as gallstones and kidney stones.
2. In this study, we investigated the prevalence of gallstones and kidney stones in patients with intestinal BD and analyzed various risk factors associated with each.
3. This study aims to identify factors influencing the occurrence of gallstones and kidney stones in patients with intestinal BD, offering insights for predicting and managing related complications
Acknowledgments
Authors are grateful to the patients and colleagues for their valuable contributions to this study.
Notes
CRedit authorship contributions
Jaewon Song: data curation, formal analysis, writing - original draft, writing - review & editing; Soo Jung Park: resources, investigation; Jae Jun Park: resources, investigation; Tae Il Kim: resources, investigation; Jihye Park: conceptualization, writing - review & editing, supervision; Jae Hee Cheon: conceptualization, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None