Timing and predictors of death during treatment in patients with multidrug/rifampin-resistant tuberculosis in South Korea
Article information
Abstract
Background/Aims
This study aimed to investigate the timing and predictors of death during treatment among patients with multidrug/rifampin-resistant tuberculosis (MDR/RR-TB) in South Korea.
Methods
This was a retrospective cohort study that included MDR/RR-TB cases notified between 2011 and 2017 in South Korea.
Results
Among 7,226 MDR/RR-TB cases, 699 (9.7%) died at a median of 167 days (IQR 51–358 d) from the initiation of MDR-TB treatment. The cumulative proportion of all-cause death was 35.5% at 90 days and 52.8% at 180 days from treatment initiation. TB-related deaths occurred at a median of 133 days (IQR 32–366 d), which was significantly earlier than the median of 184 days (IQR 68–356 d) for non-TB-related deaths (p = 0.002). In a multivariate analysis, older age was the factor most strongly associated with death, with those aged ≥ 75 years being 68 times more likely to die (aHR 68.11, 95% CI 21.75–213.26), compared those aged ≤ 24 years. In addition, male sex, comorbidities (cancer, human immunodeficiency virus, and end stage renal disease), the lowest household income class, and TB-specific factors (previous history of TB treatment, smear positivity, and fluoroquinolone resistance) were identified as independent predictors of all-cause death.
Conclusions
This nationwide study highlights increased deaths during the intensive phase and identifies high-risk groups including older people and those with comorbidities or socioeconomic vulnerabilities. An integrated and comprehensive strategy is required to reduce mortality in patients with MDR/RR-TB, particularly focusing on the early stages of treatment and target populations.
INTRODUCTION
Tuberculosis (TB) remains one of the deadliest infectious diseases caused by a single agent. Although the introduction of effective anti-TB chemotherapy has led to a decrease in TB deaths, global TB deaths remain high. In 2021, an estimated 1.6 million TB deaths occurred worldwide, making it the 13th leading cause of death globally and the second leading cause of death from a single infectious agent, following coronavirus disease 2019 (COVID-19) [1]. In South Korea, the number of TB deaths in 2021 was 1,430 (2.8 per 100,000), showing a 5.5% increase compared to that in 2020 (1,356 persons, 2.6 per 100,000), ranking third among Organization for Economic Co-operation and Development (OECD) countries [2].
The risk of death in patients with multidrug/rifampin-resistant tuberculosis (MDR/RR-TB) is notably higher than that in patients with drug-susceptible TB [3–5]. Identifying the timing and predictors of death during treatment is essential to determine the optimal timing and target populations for effective interventions. Several studies have reported various risk factors associated with mortality in patients with MDR/RR-TB [6,7]. Considering the diverse situations in different countries, it is crucial to identify country-specific risk factors. While some studies have highlighted the risk factors for TB deaths in South Korea [8–11], the majority have focused on drug-susceptible TB and do not represent nationwide data.
To address this gap, we constructed an integrated TB database by linking three national databases [12] and reported the nationwide treatment outcomes of patients with MDR/RR-TB notified between 2011 and 2017 [13,14]. Our findings revealed that death during treatment constituted the largest proportion of unfavorable outcomes, presenting a new challenge in the management of MDR/RR-TB in South Korea [13]. Subsequently, we investigated the timing and predictors of death during treatment of patients with MDR/RR-TB in South Korea
METHODS
Study design and population
This retrospective cohort study included patients with MDR/RR-TB notified between January 1, 2011 and December 31, 2017, which were extracted from the Korean Tuberculosis and Post-Tuberculosis (TB-POST) cohort. The TB-POST cohort was constructed by linking the following three national databases: 1) the Korean Tuberculosis Surveillance System (KTBS) between 2011 and 2018, 2) the National Health Insurance Database between 2006 and 2018, and 3) the Causes of Death Statistics database between 2011 and 2018 [12]. Patients with treatment outcomes assigned to death during treatment were selected.
Definition and measurement
MDR-TB was defined as TB that is resistant to at least isoniazid (INH) and rifampicin (RIF) [15]. Extensively drug-resistant TB (XDR-TB) was defined based on the criteria used during the study period: TB resistant to at least INH and RIF, in addition to any fluoroquinolone (FQ) and at least one of the injectable second-line drugs (amikacin, kanamycin, or capreomycin) [15]. Pre-XDR-TB was defined as TB with resistance to INH and RIF, and either a FQ or a second-line injectable agent, but not both.
Treatment outcomes were defined according to World Health Organization (WHO) criteria [15]. An attending physician assigned the treatment outcomes to each patient and registered them with the KTBS. Death was defined as the death of a patient with TB due to any reason during treatment. Death cases were recorded in the KTBS as either a TB-related death or a non-TB-related death. Household income was classified into quintiles (1 = lowest, 5 = highest), according to the national health insurance premium. Medical aid beneficiaries were classified as group 0.
The regions were categorized into metropolitan that included a special city (Seoul), metropolitan cities (Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and special self-governing city (Sejong), and others that included provinces other than metropolitan areas. Comorbidities were defined using claim data one year before and after diagnosis of TB. Diabetes mellitus (DM) was defined as at least three claims of International Classification of Diseases 10th Revision (ICD-10) code for DM. The presence of human immunodeficiency virus (HIV) and cancer were defined by one or more claims of ICD-10 code for each disease. End stage renal disease (ESRD) was defined cases who submitted claims for any procedure or material associated with either hemodialysis or peritoneal dialysis combined with the ICD-10 code for ESRD.
Statistical analysis
Continuous variables were presented as mean ± standard deviation if the variable was normally distributed; otherwise, they were described as median (interquartile range [IQR]). Categorical variables were expressed as number (percentage). Student’s t-test or Mann–Whitney U test was used to compare continuous variables. The chi-squared test or Fisher’s exact test was used to compare categorical variables. The Cox proportional hazards model was used to calculate the hazard ratios (HRs) for the predictors of all-cause mortality. In this analysis, the death group was compared with the combined group in terms of success and failure. Variables with p values < 0.2 on univariate analysis were entered into the multivariate models. All p values were two-tailed, and a p value of < 0.05 was deemed statistically significant. All statistical analyses were performed using STATA/MP version 17 (StataCorp LLC, College Station, TX, USA).
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board of the National Evidence-based Healthcare Collaborating Agency (NECAIRB19-008-1). Informed consent was waived because of the retrospective study using public de-identified data.
RESULTS
Trend in fatality
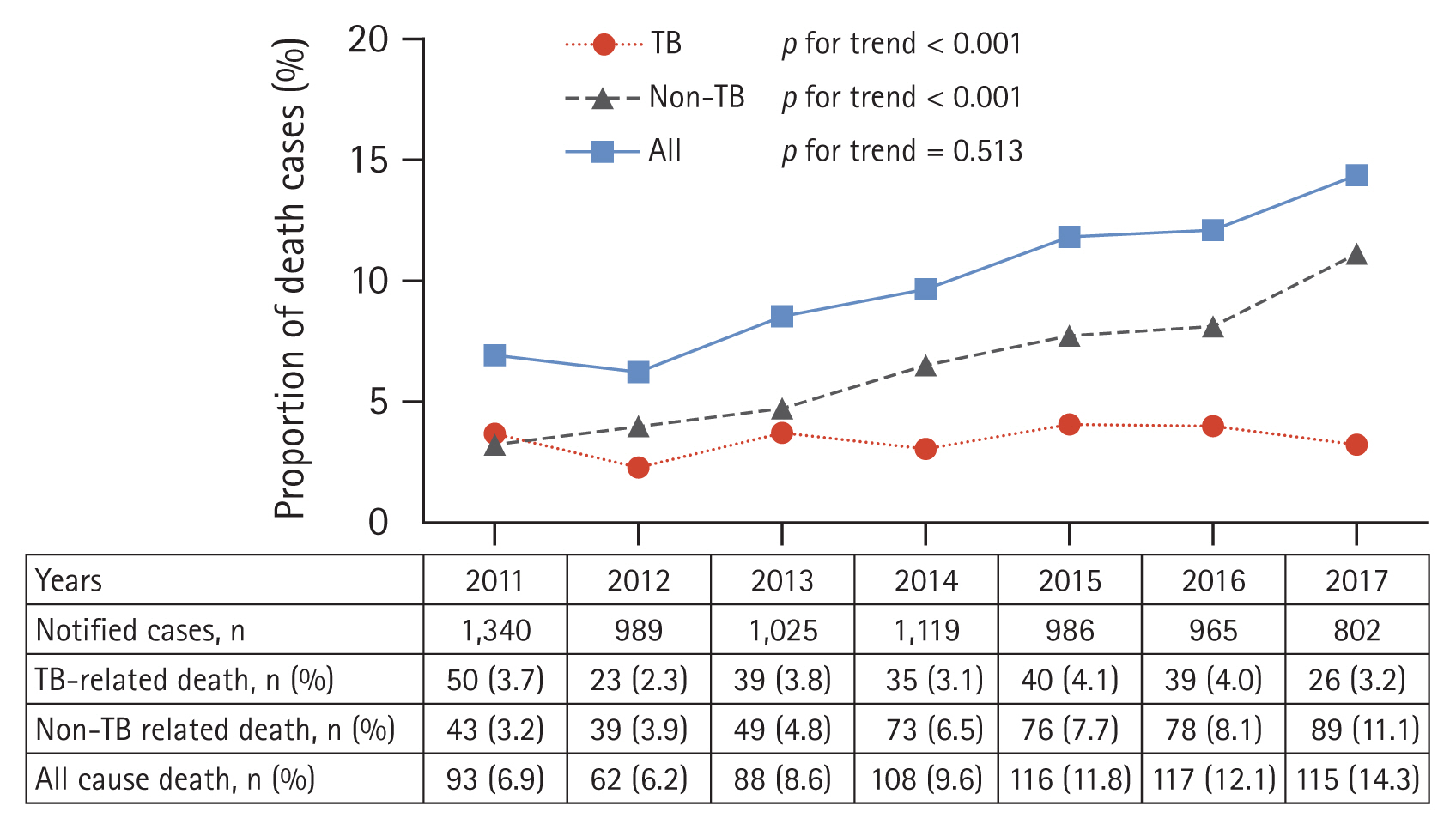
In total, 7,226 MDR/RR-TB cases were identified in the integrated TB database. Among them, 699 (9.7%) died during TB treatment, with 252 (36.1%) classified as TB-related deaths and 447 (63.9%) classified as non-TB-related deaths. The all-cause death rate during treatment increased gradually from 6.9% in 2011 to 14.3% in 2017 (p for trend < 0.001) (Fig. 1). The TB-related death rate did not significantly changed (p for trend = 0.513); however, the non-TB-related death rate gradually increased from 3.2% in 2011 to 11.1% in 2017 (p for trend < 0.001).
Baseline characteristics
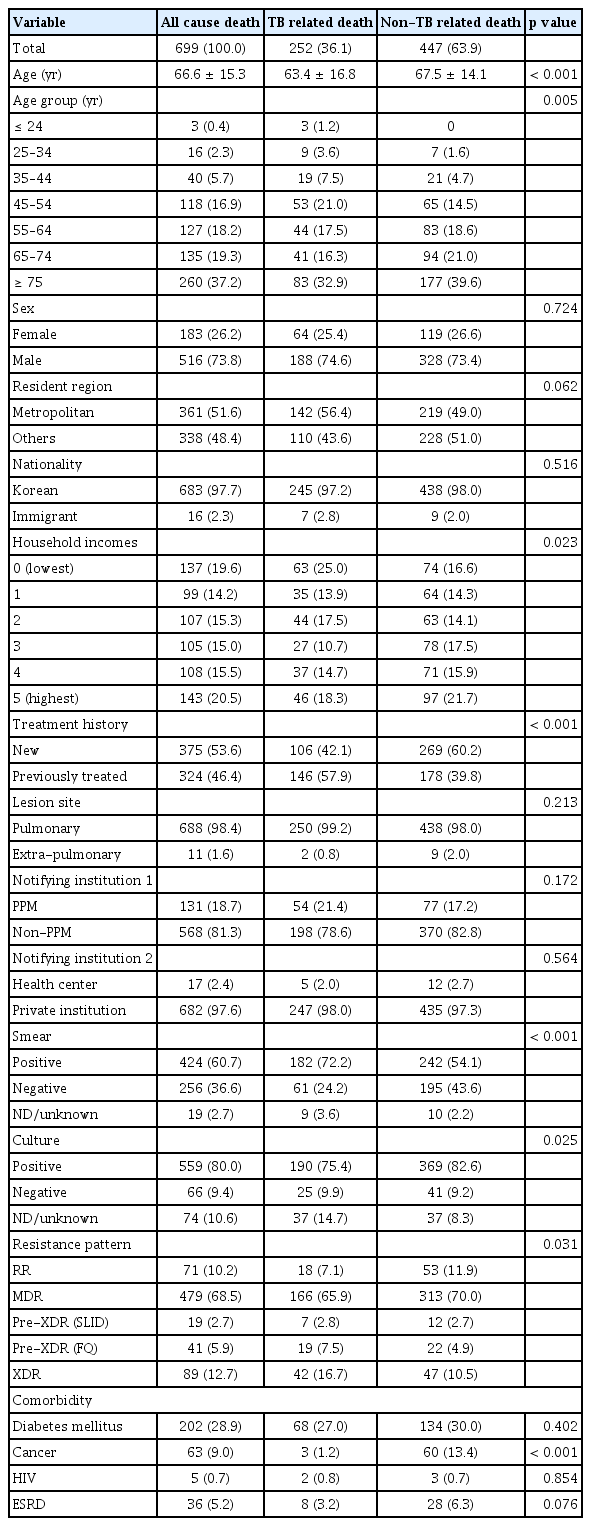
Table 1 presents the baseline and clinical characteristics of the patients who died during treatment. The TB-related death group had a higher proportion of the lowest income class (25.0% vs. 16.6%, p = 0.007), previously treated cases (57.9% vs. 39.8%, p < 0.001), smear-positive cases (72.2% vs. 54.1%, p < 0.001), and FQ-resistant MDR-TB cases (24.2% vs. 15.4%, p = 0.004) than the non-TB-related death group. In contrast, the non-TB-related death group had a higher mean age (67.5 yr vs. 63.4 yr, p < 0.001) and a higher proportion of cancer (13.4% vs 1.2%, p < 0.001) as a comorbidity than the non-TB-related death group.
Timing of deaths
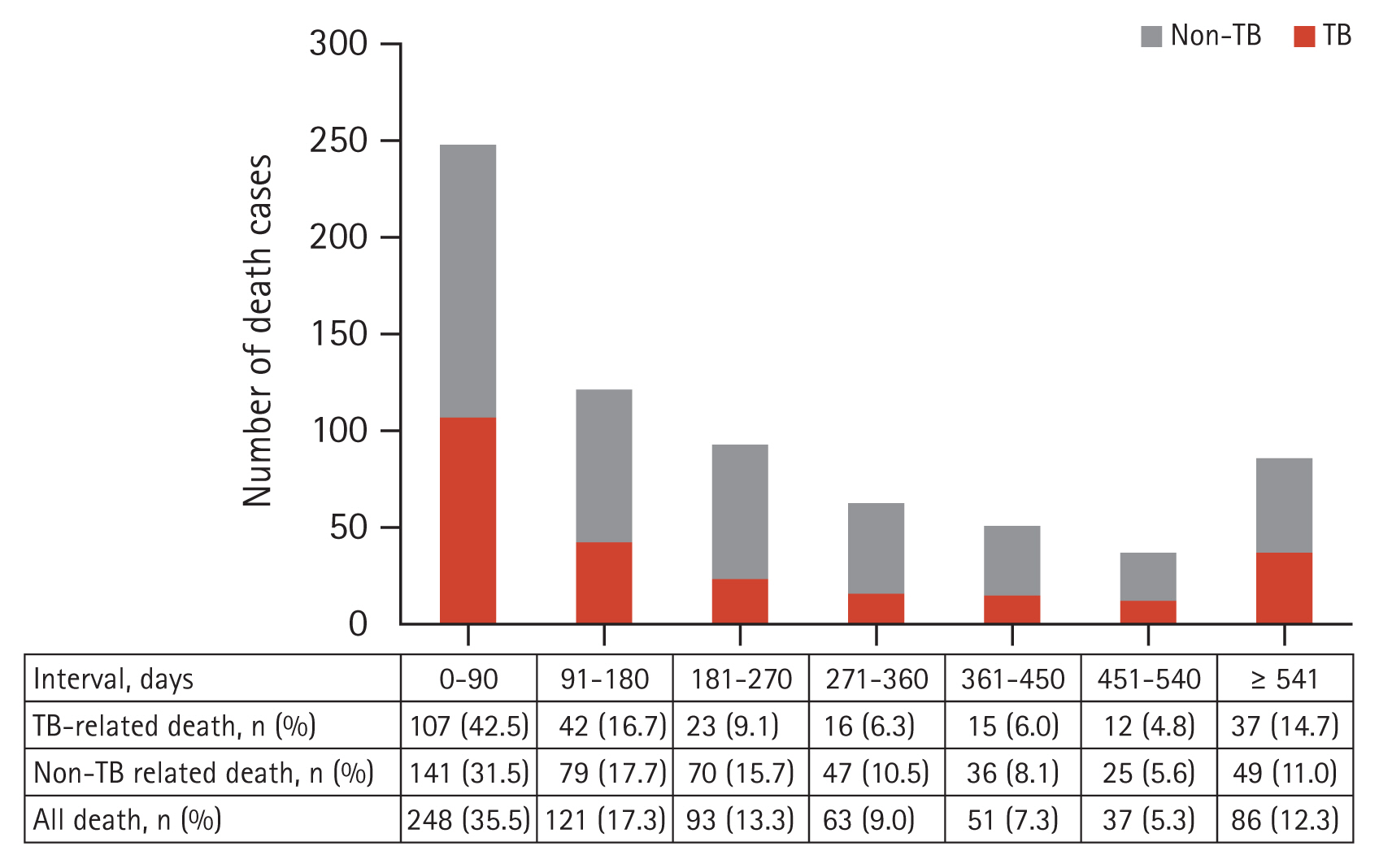
A total of 699 patients died at a median of 167 days (IQR 51–358 d) from the initiation of MDR-TB treatment. TB-related deaths occurred at a median of 133 days (IQR 32–366 d), which was significantly earlier than the median of 184 days (IQR 68–356 d) in the non-TB-related death group (p = 0.002). The cumulative proportion of all-cause deaths was 35.5% at 90 days and 52.8% at 180 days after treatment initiation (Fig. 2). The cumulative percentage of deaths at 180 days was 59.2% in the TB-related death group and 49.2% in the non-TB-related death group.
Predictors of all-cause deaths
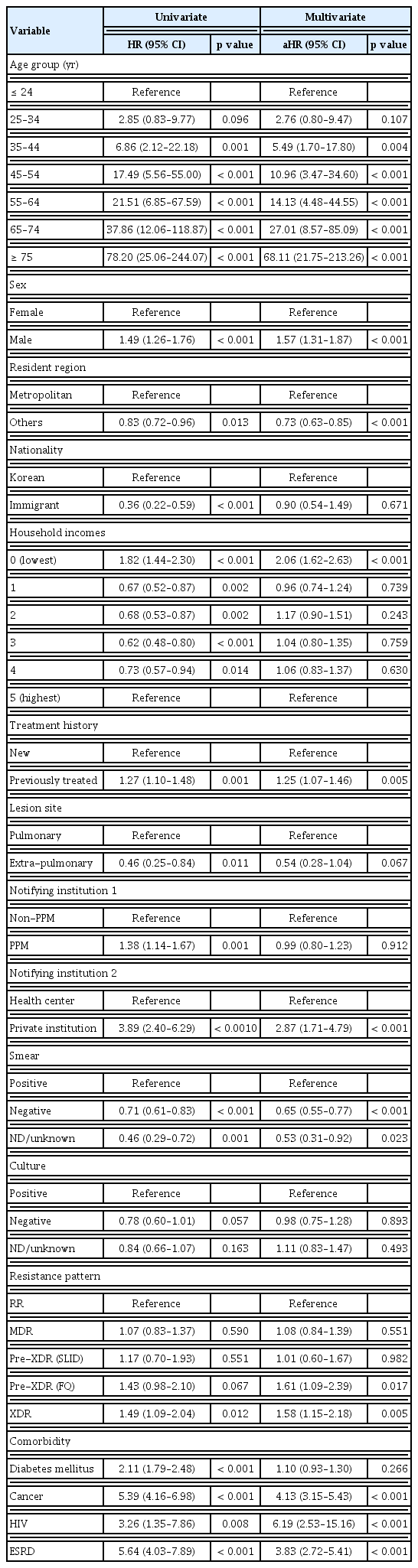
Table 2 shows predictors of all-cause deaths. In the multivariate analysis, older age, male sex (adjusted hazard ratio [aHR] 1.57, 95% confidence interval [CI] 1.31–1.87, p < 0.001), comorbidities (HIV [aHR 6.19, 95% CI 2.53– 15.16, p < 0.001], cancer [aHR 4.13, 95% CI 3.15–5.43, p < 0.001], and ESRD [aHR 3.83, 95% CI 2.72–5.41, p < 0.001]), the lowest household income class (aHR 2.06, 95% CI 1.62–2.63, p < 0.001), previous history of TB treatment (aHR 1.25, 95% CI 1.07–1.46, p = 0.005), smear positivity, pre-XDR-TB(FQ) (aHR 1.61, 95% CI 1.09–2.39, p = 0.017), and XDR-TB (aHR 1.58, 95% CI 1.15–2.18, p = 0.005) were identified as independent predictors of all-cause death.
DISCUSSION
This study aimed to investigate the timing and predictors of death during treatment among patients with MDR/RR-TB in South Korea. Approximately 10% of patients with MDR/RR-TB died during their treatment, and nearly half of these deaths occurred within 6 months of initiating MDR-TB treatment. This study also identified various predictors of death during treatment, encompassing demographic factors (old age and male sex), clinical factors (comorbidities), TB-specific factors (smear positivity, FQ resistance, and history of previous TB treatment), and socioeconomic factors (belonging to the lowest income class). This study emphasizes the need for an integrated and comprehensive strategy to reduce mortality in patients with MDR/RR-TB, particularly focusing on the early stages of treatment and vulnerable populations.
The study revealed that deaths occurred more frequently in the intensive phase, but were consistently observed throughout the entire treatment duration. TB-related deaths occurred earlier (median, 4.4 mo) compared to non-TB-related deaths (median, 6.1 mo). This aligns with the findings of a cohort study on drug-susceptible TB in South Korea that showed that many TB-related deaths occurred during the intensive phase [8]. Therefore, interventions aimed at reducing mortality should prioritize the early stages of treatment, emphasizing strategies such as early diagnosis and prompt initiation of appropriate treatment. This includes active case findings in high-risk populations and the expanded use of rapid molecular drug susceptibility testing (DST).
The distribution of the timing of deaths in this study also highlights the need for new treatment strategies beyond early detection and treatment. In a retrospective MDR/RR-TB cohort study using new drugs in South Korea [16], only age and low body mass index were found to be independent predictors of treatment outcomes, whereas traditional TB-specific factors (smear positivity, previous TB treatment, presence of cavity, drug resistance patterns) did not significantly predict treatment outcomes. This finding suggests that effective treatment with new drugs may mitigate the impact of TB-specific factors on treatment outcomes. However, despite achieving a high treatment success rate of 79.2%, the mortality rate remained high at 11.9% [16]. Therefore, simply incorporating new drugs into conventional longer regimens may not be sufficient to reduce mortality rates. Considering the cause and timing distribution of deaths shown in this study, the introduction of new, shorter regimens of 6–9 months [17,18] is expected to be an effective strategy to reduce mortality in patients with MDR/RR-TB.
This study revealed various risk factors associated with deaths, including demographic, clinical, and socioeconomic factors, which align with the findings of previous studies [6]. The risk factors associated with a higher hazard ratio in this study were age and comorbidities. Age is likely to have a great impact on death during treatment, with patients aged ≥ 65 years contributing to 65% of total deaths. The risk of death increased proportionally with age, reaching a 68 times higher risk for patients aged ≥ 75 years than those ≤ 24 years. Risk factors associated with death during TB treatment varied by region [19]. HIV positivity is the primary risk factor for death in countries with a high incidence of TB and prevalence of HIV [7,19]. However, age and non-infectious comorbidities were the main risk factors for death in regions with a low incidence of TB and HIV [19–21], as shown in this study.
Older patients face a higher risk of both incidence of TB and TB-related death, primarily because of immunosenescence, comorbidities, and an increased susceptibility to adverse reactions during treatment [22,23]. Although TB in older patients has traditionally been considered a concern in developed countries [24], it has now evolved into a global challenge with increasing life expectancy [22]. Notably, South Korea, which has one of the fastest-growing older populations globally, has encountered challenges with TB in older population [25,26]. A tailored strategy that encompasses initiatives, such as the implementation of routine TB screening at aged-care facilities, the establishment of age-friendly infrastructure and services, and the integration of TB and non-communicable diseases services, has the potential to significantly improve TB management among older adults [27].
This study identified that comorbidities and the lowest income level as independent predictors of death, which was consistent with previous studies [28,29]. In contrast to age, comorbidities and socioeconomic factors may be modifiable. Various risk factors from demographic, clinical, and socioeconomic perspectives contribute to TB-related deaths, and these factors are interconnected. For example, lower socioeconomic groups face a higher risk of malnutrition, engage in less healthy behaviors (such as smoking and alcohol abuse), are less likely to seek healthcare, and are more likely to have comorbidities [28]. Therefore, a comprehensive and integrated approach is required to reduce mortality in patients with MDR/RR-TB.
The risk of death among TB patients has generally been higher in rural areas than in urban areas, likely due to differences in the accessibility and quality of health care [30]. The lower risk of death in non-metropolitan areas compared to metropolitan areas suggests that there may not be a significant disparity in healthcare access in South Korea. Furthermore, this suggests that high-risk groups, such as the homeless and single-room occupants with limited medical accessibility, may be more prevalent in metropolitan areas rather than in non-metropolitan areas.
To our knowledge, this is the first study to evaluate the timing and predictors of death in a nationwide MDR/RR-TB cohort in South Korea. The strength of this study is that it was a population-based study covering almost all MDR/RR-TB cases notified in South Korea. However, the retrospective nature of this study and its reliance on routinely collected health data introduced several limitations. First, the cause of death might not be accurate. In the KTBS, the cause of death was determined by an attending physician or TB nurse based on the death certificate. This was not been confirmed through autopsy or alternative methods, potentially leading to a reporting bias. However, this potential misclassification did not affect the study results because we analyzed the risk factors for all-cause death. A verification process to determine the exact cause of death and subsequent analysis of the predictive factors for TB-related death is necessary. Second, regimen-related factors and adverse drug reactions were not included in the assessment of risk factors for mortality. Third, this study did not adequately capture recent advancements in MDR-TB treatment. Several studies conducted in other countries have reported that the use of new drugs contributes to a reduction in TB deaths [31,32]. This study has limitations in determining the impact of new drugs on mortality because the use of the new drug was limited during the study period. Therefore, further studies using nationwide data are required.
In conclusions, this nationwide study highlighted the increased deaths during the intensive phase and identified high-risk groups, including older people and those with comorbidities or socio-economic vulnerabilities. A comprehensive strategy, including active case finding, early detection with molecular DST, introduction of new, shorter regimens, comorbidity management, and socioeconomic support, is crucial for reducing MDR/RR-TB mortality in South Korea.
KEY MESSAGE
1. More than half of deaths in MDR/RR-TB patients occurred during the intensive phase of treatment.
2. Older age, comorbidities, and socioeconomic vulnerabilities increased the risk of death during treatment.
3. A comprehensive strategy targeting vulnerable populations and the early stage of treatment is needed to reduce mortality rates in MDR/RR-TB patients.
Acknowledgments
This study used the National Health Information Database (NHIS-2019-1-662) of the National Health Insurance Service (NHIS).
Notes
CRedit authorship contributions
Eunjeong Son: methodology, investigation, data curation, writing - original draft; Hongjo Choi: methodology, investigation, formal analysis, writing - review & editing, funding acquisition; Jeongha Mok: methodology, investigation, data curation, writing - review & editing; Young Ae Kang: methodology, investigation, data curation, writing - review & editing; Dawoon Jeong: investigation, data curation, formal analysis, writing - review & editing; Doosoo Jeon: conceptualization, methodology, validation, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was financially supported by the National Evidence-based Healthcare Collaborating Agency, funded by the Ministry of Health and Welfare (grant No. NC19-002, NC20-003, and NC21-001).