Screening and diagnosis of atrial fibrillation using wearable devices
Article information
Abstract
In recent years, the development and use of various devices for the screening of atrial fibrillation (AF) have significantly increased. Such devices include 12-lead electrocardiogram (ECG), photoplethysmography systems, and single-lead ECG and ECG patches. This review outlines several studies that have focused on the feasibility and efficacy of such devices for AF screening, and summarizes the risks and benefits involved in the initiation of anticoagulant therapy after early detection of AF. We also describe several ongoing trials on unresolved issues associated with AF screening. Overall, this review provides a comprehensive summary of the current state of AF screening and its implications for patient care.
INTRODUCTION
Atrial fibrillation (AF) is a common cardiac arrhythmia associated with increased risk for ischemic stroke, heart failure, cognitive impairment, and all-cause mortality [1-3]. Cardioembolic stroke associated with AF is usually severe, has a high risk of recurrence, and can result in fatal outcomes or permanent disability [4,5]. Appropriate anticoagulant therapy is effective for preventing cardioembolic stroke in patients with AF [6,7]. However, AF can sometimes be transient or asymptomatic, and stroke can be its first manifestation [8-10]. This makes early diagnosis and treatment difficult.
Early diagnosis of AF allows for implementation of oral anticoagulant therapy and prevents the unwanted consequences of undetected disease; thus, AF screening in atrisk populations may be beneficial. However, the clinical benefits of AF screening in asymptomatic patients remain unclear [11-15]. Recommendations for AF screening are inconsistent even in currently established guidelines [2,16,17]. The European Society of Cardiology recommends performing opportunistic screening for AF via pulse measurement or electrocardiogram (ECG) rhythm strips in patients aged ≥ 65 years as a Class I recommendation [2]. However, the United States Preventive Services Task Force concluded that there is insufficient evidence to assess the balance of the benefits and harms of AF screening, as indicated by their assignment of an “I statement” [16].
This article summarizes the systems used for AF screening and how they affect hard outcomes when AF screening is appropriately performed and managed.
PREVIOUS STUDIES ON AF SCREENING
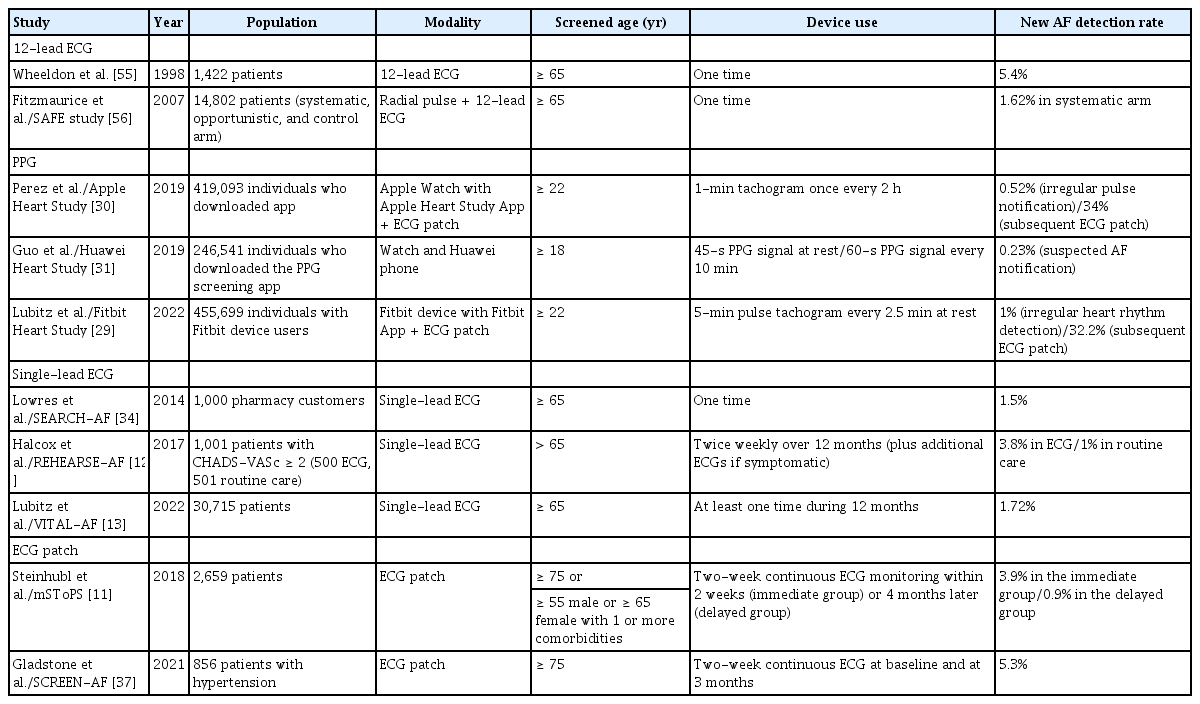
Various systems are used for AF screening. Traditional modalities include one-time pulse measurement and 12-lead ECG. However, these mainly identify persistent or high-burden paroxysmal AF [18]. Continuous ECG monitoring using a Holter device, ECG patches, or implantable cardiac devices have better diagnostic rates for paroxysmal arrhythmia. New systems, such as photoplethysmography (PPG) devices, single-lead ECG devices, and other sensors integrated into smartphone applications [19-21], have the advantages of being cost-effective, handheld, and wearable. Table 1 summarizes the findings of trials on the feasibility of AF screening.
Holter monitoring
Holter monitoring is a conventional method of AF screening. Three to five ECG electrodes are connected to yield two ECG vectors and a third derived electrogram [22]. Patients record their symptoms and the time at which the symptoms occur. Holter monitoring is used to evaluate the average heart rate, quantify the number of atrial and ventricular ectopics, and determine the presence of AF. The duration and burden of AF as well as the pattern of initiation and its termination can be determined by Holter monitoring. However, screening for paroxysmal AF using a Holter monitor is not suitable for asymptomatic individuals because of its low diagnostic yield of detection [23-25]. One study evaluated the diagnostic yield of paroxysmal AF in patients of advanced age (≥ 65 yr) undergoing primary care via Holter monitoring and intermittent single-lead ECG [24]. Patients with a median of 8 days of Holter monitoring had a low diagnostic yield of paroxysmal AF (1.5%; 95% confidence interval [CI], 0.4–3.8%).
PPG devices
PPG devices measure fluctuations in the blood volume within capillaries and translate this volume into a pulse wave. A PPG device emits a continuous stream of photons through the skin, whereas a photodetector measures the changing intensities of the reflected photons [26-28]. Identification of pulse irregularities using PPG has the potential to identify AF. PPG offers continuous monitoring for arrhythmia detection, is cost-effective, and is simple. However, it is vulnerable to motion artifacts, which can reduce the accuracy of AF detection [29], and it does not allow for p-wave analysis.
Several studies have shown that wearable devices can facilitate the diagnosis of AF in asymptomatic patients. The Apple Heart Study was a single-arm prospective study that enrolled 419,093 participants using the Apple Watch with the Apple iPhone app [30]. During a 117-day follow-up period, 0.52% of the participants received notification of an irregular pulse. Of 450 participants who underwent 1-week ECG patch monitoring after notification, 34% had AF. The Huawei Heart Study monitored the pulse rhythm of 187,912 participants via PPG monitoring, of whom 424 (0.23%) received a “suspected AF” notification [31]. Among them, 87% were confirmed to have AF. Of those diagnosed with AF, 95.1% were enrolled in a program of integrated AF management via a mobile app. The Fitbit Heart Study was a prospective remote clinical trial that enrolled 455,699 participants, of whom 1% reported an irregular heart rhythm. Among the 1,057 participants who received notification, 32.2% were found to have AF by subsequent ECG patch monitoring [29].
Single-lead ECG
Single-lead ECG uses a 30–60 seconds rhythm strip with two different polarities. Smartwatches and wristbands have recently become useable as single-lead ECGs. For such devices, a contralateral finger is positioned to the side of the device, and the back is set against the wrist to serve as the positive electrode [32]. While single-lead ECG is feasible for detecting AF, its accuracy for identifying more complex arrhythmias and interval abnormalities is comparatively low [33].
Many studies have demonstrated the feasibility of AF screening using single-lead ECG. The Screening Education and Recognition in Community Pharmacies of Atrial Fibrillation (SEARCH-AF) study investigated community-based AF screening using iPhone ECG [34]. Of 1,000 participants aged > 65 years, 1.5% were found to have new-onset AF. The study showed that the AF screening was both feasible and cost-effective. Among participants who underwent biweekly testing using the AliveCor KardiaMobile monitor in the Assessment of Remote Heart Rhythm Sampling Using the AliveCor Heart Monitor to Screen for Atrial Fibrillation (REHEARSE-AF) study, AF was detected four times more frequently in an active than in a control group [12].
ECG patch
Continuous ECG-based methods exhibit higher sensitivity for AF screening than other modalities [35]. Compared to Holter monitoring, ECG patches have the advantage of being easier to apply and less likely to cause skin problems, resulting in reduced discomfort during extended examination. Kwon et al. [36] compared ECG patches and Holter monitoring and reported that the former can be considered as an alternative to the latter; there was remarkable consistency between the two devices in terms of total QRS complexes, premature ventricular beats, supraventricular ectopic beats, and heart rates. Notably, however, the limitations of ECG patches include the use of single-lead ECG, which is less effective for distinguishing p-waves or filtering out noise. Furthermore, there are concerns regarding potential degradation in the quality of analysis because of the substantial human resources required for the analysis of long-term records.
The SCREEN-AF study evaluated two home-based AF screening interventions in individuals aged > 75 years with hypertension and without a previous AF diagnosis [37]. A continuous ECG patch group exhibited a 10-fold increase in AF detection compared to a group in which home blood pressure monitoring was employed. The mHealth Screening to Prevent Strokes trial investigated the efficacy of a wearable ECG patch for detecting AF and its associated clinical outcomes [11]. Among 2,659 participants, immediate monitoring using the wearable ECG patch identified more cases of AF than delayed monitoring. Although monitored individuals more often utilized health care services, the risk for AF-related emergency department visits and hospitalizations did not significantly differ between active-monitoring and control groups.
The diagnostic yield of AF increases as the frequency and duration of AF screening increases [13,38]. Single-lead ECG utilizes a single measurement and is performed when the patient is symptomatic. Therefore, compared to single-lead ECG, the use of ECG patches for continuous monitoring increases the rate of AF detection [39].
CARDIAC IMPLANTABLE ELECTRONIC DEVICES (CIEDs)
The types of AF detected via opportunistic screening in primary care must be distinguished from paroxysmal subclinical AF detected using implanted cardiac monitors or wearable devices [18]. Continuous monitoring using CIEDs has shown that atrial tachyarrhythmias, defined as atrial high-rate episodes (AHREs), occur in approximately one-third of patients with CIEDs [40,41]. An observational study on AHREs detected via CIEDs reported that AHREs lasting > 6 minutes increased the risk of stroke [42]. The risk of ischemic stroke increased with longer durations of AHREs, particularly those lasting > 24 hours [43-45]. The LOOP study enrolled individuals without AF aged 70–90 years with at least one of the following risk factors for stroke: hypertension, diabetes, prior stroke, and heart failure. In an implantable loop recorder group, anticoagulation was recommended if AF lasted for ≥ 6 minutes. The rates of AF detection and initiation of anticoagulation were significantly higher in the implantable loop recorder group than in controls. Nonetheless, there was no disparity in the reduction of the risk of stroke or systemic arterial embolism between the two groups [46]. The recently published Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High-Rate Episodes study enrolled patients aged ≥ 65 years who had AHREs lasting > 6 minutes as detected using implantable devices and exhibited at least one risk factor for stroke [47]. Compared to placebo, the use of edoxaban in patients with AHREs did not significantly reduce the risk of the composite outcome involving cardiovascular death, stroke, or systemic embolism but increased the risk of composite death or major bleeding. However, the recently published Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Subclinical Atrial Fibrillation study showed that compared to aspirin, apixaban reduced the risk of stroke or systemic embolism but increased the risk of major bleeding [48]. These studies raise questions regarding the appropriate duration of AHREs for diagnosis and the need to initiate anticoagulant therapy.
RISKS AND BENEFITS OF AF SCREENING AND APPROPRIATE MANAGEMENT
The potential impact of anticoagulant treatment initiation immediately after AF screening on various outcomes, such as the risk of stroke or mortality, remains ambiguous. Research outcomes regarding this issue have yielded conflicting results.
In the REHEARSE-AF study, AF was diagnosed in 19 patients in a single-lead ECG group but in only 5 patients in a control group [12]. Despite the higher AF detection rate, the overall incidence of stroke or transient ischemic attack was similar in both groups. The STROKESTOP study, a multicenter, parallel-group, unmasked randomized controlled trial, enrolled patients aged 75–76 years for AF screening using a single-lead ECG device (Zenicor) twice daily for 2 weeks, contrary to routine care [14]. The study explored the initiation of anticoagulation therapy after AF detection. After a median follow-up period of 6.9 years, the study exhibited significantly fewer primary outcomes (including ischemia or hemorrhagic stroke, systemic embolism, serious bleeding, and death) in an intervention group than in controls. The Mobile AF Application (mAFA) trial assessed the impact of integrated management based on a mAFA compared to usual care. An integrated care approach decreased the risk of a composite outcome involving ischemic stroke/systemic thromboembolism, death, and rehospitalization [15].
Several studies have demonstrated the cost-effectiveness of AF screening. The SEARCH-AF study reported an incremental cost-effectiveness ratio of $4,066 per quality-adjusted life-year (QALY) gained [34]. In the STROKESTOP study, the incremental cost was €1.77 million lower in a screening invitation group than in controls, which indicated that AF screening in an elderly population is cost-effective [49]. Chen et al. [50] evaluated the cost-effectiveness of AF screening using wrist-worn wearable devices, including watch or band PPG, and traditional modalities, including pulse measurement and 12-lead ECG [50]. The wrist-worn wearable devices exhibited additional QALY compared to no screening. In addition, they were the most cost-effective among the modalities, showing an incremental cost-effectiveness ratio of $57,894 per QALY.
UNMET NEEDS
Several issues persist in the realm of AF screening. First, the target population for AF screening needs to be determined. Second, the optimal frequency and monitoring duration require consideration. Finally, it is necessary to establish a threshold of the AF burden requiring treatment. Various guidelines recommend opportunistic screening for individuals aged ≥ 65 years [2,17,51]. Systematic ECG screening is recommended in individuals aged ≥ 75 years and those at high risk for stroke, as per the Class IIa recommendation in the European Society of Cardiology guidelines [2].
The increasing use of wearable devices has sparked a growing interest in consumer-led screening. Both intermittent and continuous monitoring of various durations, as opposed to single recordings, enhance the diagnostic yield of AF. However, repeated examinations also increase the risk of detecting low-burden AF, which typically has a benign prognosis. In addition to the findings from the mAFA trial [15], further studies are warranted to ascertain the impact of AF screening on hard clinical outcomes for the general population. Another issue is false-positive AF, which may result in complications associated with unnecessary treatments, such as heightened patient anxiety, increased financial costs, and anticoagulation-related complications (e.g., bleeding and diminished quality of life) [52]. Furthermore, unresolved ethical, legal, and privacy concerns are associated with consumer-led screening [39]. The balance between the potential benefits and risks of consumer-led screening must be delineated.
Several ongoing trials are investigating the association between AF screening and patient outcomes. The Reducing Stroke by Screening for Undiagnosed Atrial Fibrillation in Elderly Individuals trial, a randomized trial utilizing long-term patch monitoring, aims to determine whether AF screening results in reduced stroke incidence [53]. In addition, the ongoing Heartline Study will evaluate the impact of the smartwatch irregular rhythm notification algorithm and inbuilt ECG on cardiovascular events [54].
CONCLUSION
The application of AF screening in clinical practice is expected to increase. This article discusses the significance of AF screening and its impact on patient outcomes. Wearable devices have shown promise in improving AF detection rates compared to traditional modalities. However, research findings associated with the benefits of early AF diagnosis using wearable devices and anticoagulant therapy are controversial. Several trials are ongoing to elucidate the impact of this issue. Challenges in AF screening include defining the target population, monitoring frequency, and establishing AF burden thresholds for treatment. With the increase in consumer-led screening using wearable devices, ethical and privacy issues will also need to be addressed in the future.
Notes
CRedit authorship contributions
Yoon Jung Park: data curation, formal analysis, writing - original draft, writing - review & editing; Myung Hwan Bae: conceptualization, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None