Principles and practices of antimicrobial stewardship programs in Korea
Article information
Abstract
This review addresses the escalating challenge posed by antibiotic resistance, highlighting its profound impact on global public health, including increased mortality rates and healthcare expenditures. The review focuses on the need to adopt the One Health approach to effectively manage antibiotic usage across human, animal, and environmental domains. Antimicrobial stewardship programs (ASPs) are considered as comprehensive strategies that encompass both core and supplementary initiatives aimed at enhancing prudent antibiotic use. The 2021 “Guidelines on Implementing ASP in Korea” introduced such strategies, with a strong emphasis on fostering multidisciplinary and collaborative efforts. Furthermore, the “Core Elements for Implementing ASPs in Korean General Hospitals,” established in 2022, provide a structured framework for ASPs, delineating leadership responsibilities, the composition of interdisciplinary ASP teams, a range of interventions, and continuous monitoring and reporting mechanisms. In addition, this review examines patient-centric campaigns such as “Speak Up, Get Smart” and emphasizes the pivotal role of a multidisciplinary approach and international cooperation in addressing the multifaceted challenges associated with antibiotic resistance.
INTRODUCTION
Since Alexander Fleming’s discovery of penicillin, antibiotics have been essential in treating bacterial infections such as pneumonia and strep throat. Initially termed “magic bullets” by Paul Ehrlich owing to their ability to target bacteria without affecting healthy cells, antibiotics have been widely used in the mid-20th century. However, misuse and overuse of antibiotics have contributed to the emergence of antibiotic-resistant bacteria, posing a significant threat to public health. Therefore, responsible management of antibiotics is crucial for maintaining their efficacy for future generations [1,2].
ESCALATION OF ANTIBIOTIC RESISTANCE
The continuous use of antibiotics has led to an increase in antibiotic resistance, which is considered a growing public health concern [2]. It is estimated that bacterial antimicrobial resistance (AMR) directly caused 1.27 million deaths globally in 2019 and contributed to an additional 4.95 million deaths [2,3]. This issue has substantially escalated in Korea, as evidenced by recent studies [4,5]. Resistance occurs when bacteria evolve to withstand antibiotics, thus diminishing their effectiveness. Although complete eradication of this resistance may be impossible, its impact can be mitigated through proper antibiotic use, the development of new antibiotics, and the implementation of antimicrobial stewardship programs (ASPs) [1,6,7]. It is also crucial to enhance infection control measures [2,8].
Addressing this challenge is particularly difficult with the increase in antibiotic usage [1,2]. The strategies to mitigate resistance and protect public health are multifaceted. These include educating healthcare providers and the public, establishing prescribing guidelines, advocating for prudent antibiotic use, and encouraging ongoing research [1]. A concerted effort is warranted to ensure the continued effectiveness of antibiotics and safeguard public health [1].
IMPACT OF ANTIBIOTIC RESISTANCE
Antibiotic resistance is a serious challenge to global public health, leading to increased mortality rates and additional burden on healthcare systems through prolonged treatments and rising costs. This issue has far-reaching implications, affecting both societal well-being and economic development [2,8]. If current trends persist, the number of deaths caused by antibiotic resistance is projected to reach 10 million annually by 2050, surpassing those caused by cancer [9].
AMR poses a serious threat to global health and economies. The 2014 O’Neill Review projected a global gross domestic product decline of 2–3.5% by 2050, with costs potentially reaching US$ 100 trillion due to AMR [9]. Contrarily, the 2017 World Bank report estimated a decline of 1.1–3.8% [10]. The 2018 report of the Organisation for Economic Co-operation and Development (OECD) forecasted 2.4 million deaths in Europe, North America, and Australia from 2015 to 2050, with an annual cost of US$ 3.5 billion across 33 OECD countries [11]. Sensitivity analysis revealed that with a 25% and 50% increase in AMR resistance rates, deaths could increase to 5.7 and 6.1 million, respectively, from 2020 to 2030, with economic costs escalating to US$ 167 billion and US$ 181 billion, respectively. These figures highlight the urgent need for global action against AMR [12].
In Korea, a study demonstrated the substantial impact of multidrug-resistant organisms (MDROs) revealing that bacteremia caused by five types of MDROs affected 7,979 patients, leading to 3,280 deaths [13]. These infections incurred substantial losses, amounting to approximately US$ 294.5 million, emphasizing the severe clinical and economic implications of such infections [13].
NEED TO REDUCE UNNECESSARY ANTIBIOTIC PRESCRIPTIONS
Retrospective analyses of antibiotic prescription data from US physician offices and emergency departments, aimed at determining the proportion of unnecessary prescriptions relative to national guidelines, revealed that nearly one-third of all antibiotic prescriptions may be unnecessary [14,15]. Similarly, a cross-sectional 1-day point-prevalence study in Korea found that 30.6% of all antibiotic prescriptions were inappropriate [16]. Another study reported a 14.1% prevalence of antibiotic prescriptions, of which 27.7% were inappropriate. The most frequent indication for such prescriptions was respiratory tract infection, with cephalosporins being the most commonly used antibiotics [17].
A review article incorporating data from the USA, Europe, Australia, and Korea estimated that approximately 20–55% of all antibiotic prescriptions were inappropriate [18]. These concerning statistics highlight the urgent need for ASPs to address the misuse and overuse of antibiotics. Implementing a data-driven, systematic approach is critical for the development of evidence-based prescribing guidelines. Easy access to healthcare and the aging population in Korea exacerbated the high rates of antibiotic usage, putting the nation at a high risk for antibiotic misuse and highlighting the urgent need for effective ASP implementation.
GLOBAL AND NATIONAL ACTION PLAN FOR AMR
In 2015, the World Health Assembly adopted a global action plan on AMR, which outlines five strategic objectives, including “optimizing the use of antimicrobial medicines in human and animal health” [8]. The Korean National Action Plan for AMR, launched in August 2016, aims to enhance public safety by promoting responsible antibiotic use in human (both in the community and healthcare settings) and animal domains to avoid overprescription and limit the spread of AMR [19]. Integral to the global strategy against AMR, this plan aligns with the Global Action Plan of the World Health Organization (WHO). The Korean Ministry of Health and Welfare has introduced two phases of this plan: the first (2016–2020) and the second (2021–2025) [19,20]. The second phase mainly focuses on encouraging the prudent use of antimicrobials by implementing ASPs in healthcare settings. Guidelines and core elements have been developed to foster ASPs [21,22]. Preparations are ongoing to establish an educational system for ASP team members, create a health insurance reimbursement mechanism for ASP activities, and expand ASP standards in healthcare accreditation.
Initiated in 2019, the Korea National Antimicrobial Use Analysis System (KONAS) enables healthcare facilities to gain insights into their antibiotic usage patterns, including usage by antibiotic class and application [23]. This system motivates and facilities the engagement in stewardship activities aimed at guiding appropriate use [24]. KONAS nationally monitors the quantitative use of antibiotics by individual medical facilities and aims to expand participation through institutional support [23,24].
In addition to quantitative monitoring by KONAS, a consistent qualitative evaluation of antibiotic use is crucial. Identifying inappropriate antibiotic prescriptions for infectious diseases by monitoring the quality of antibiotic use is essential for the effective implementation of antibiotic stewardship [18,25].
In addition, maintaining a national-level surveillance program, such as the Korean Global Antimicrobial Resistance Surveillance System (Kor-GLASS), is vital for monitoring the rates of antibiotic resistance [26].
WHAT ARE ASPs?
ASPs are comprehensive strategies developed using epidemiological and pharmacological data and aimed at the judicious selection, dosing, administration, and monitoring of antimicrobial agents to alleviate the emergence of drug-resistant pathogens, enhance patient safety, and optimize therapeutic outcomes while minimizing the risk of adverse events associated with antimicrobial use [1,7,22]. Central to these programs is the concept of “antibiotic stewardship,” which fundamentally focuses on the prudent use of antibiotics. The overarching goal is to preserve their effectiveness over time and minimize the risk of developing antibiotic-resistant bacteria [7,22]. For this purpose, ASPs emphasize educating healthcare providers and patients regarding proper antibiotic use, establishing evidence-based prescribing guidelines, and monitoring antibiotic usage and resistance patterns [22]. Through these measures, the program identifies improvement opportunities and interventions to optimize antibiotic usage and reduce the emergence of antibiotic resistance [1,7,22].
The effectiveness of ASPs is evaluated using various metrics: process measures such as guideline adherence, outcome measures such as reduced infection rates and resistance patterns, utilization measures that track antimicrobial use, and financial metrics reflecting cost savings [1,6,21]. Additionally, microbiological measures, quality indicators such as patient outcomes and educational metrics that evaluate professional training on antimicrobial use are crucial for evaluating ASP performance [1,6,21]. In Korea and abroad, these metrics are useful in tailoring and enhancing the program’s effectiveness across diverse healthcare settings.
ANTIMICROBIAL STEWARDSHIP IN KOREA: A DECADE OF CHANGE AND CHALLENGES
The advancement of ASPs in Korea has faced considerable challenges, such as limited time, inadequate staffing, and insufficient financial incentives. These barriers critically hinder the effective implementation and operation of these vital programs.
A comprehensive assessment across 54 Korean hospitals in 2015 revealed a marked increase in ASP adoption compared with earlier evaluations. Of these hospitals, 50 had established ASPs, mainly using preauthorization measures for antibiotic use. Despite this positive trend, there was a decrease in overall antimicrobial use. A major concern identified was the limited human resources allocated to these programs, suggesting a substantial gap in staffing necessary for an effective operation [27].
In-depth analysis revealed a trend toward the implementation of restrictive policies for specific antibiotics in Korean hospitals. While a significant majority of such hospitals (88.1%, 74 out of 84) adopted restrictive measures for certain antimicrobials, far fewer had adopted interventions for prolonged inappropriate antimicrobial use (9.5%, 8 out of 84) and strategies for transitioning from parenteral to oral administration (1.2%, 1 out of 84). These findings highlight the challenges in advancing ASPs in Korea, including the aforementioned barriers [28].
A study focused on workforce requirements for effective ASP operations in eight Korean hospitals identified a need for approximately 1.20 full-time equivalents per 100 hospital beds, with a median patient review time of 10–16 minutes. The primary function within ASPs was to review surgical prophylactic antibiotic use. [29]. However, most institutions lacked dedicated ASP personnel and often relied on one or two infectious disease specialists for program management. This highlights the urgent need for national support to strengthen and expand ASPs throughout Korea [28].
Further corroborating these challenges, another study reported that most Korean hospitals remain dependent on a limited group of infectious disease experts to manage their ASPs. Despite the widespread adoption of restrictive practices, there is a notable lack of comprehensive interventions for prolonged inappropriate antimicrobial use and strategies for transitioning from intravenous to oral antibiotics. These studies collectively emphasize the persistent issues of insufficient time, personnel, and financial resources in Korean hospitals, advocating for national-level intervention to improve the effectiveness of ASPs in Korea [30].
STRATEGIES FOR ASPs IN KOREA
ASPs in Korea include initiatives such as the “Evaluation of the Appropriate Use of Prophylactic Antibiotics,” which began in 2007. This initiative aimed to raise awareness regarding the prevention of surgical site infections and encourage healthcare institutions to improve quality of care. This approach involves regulating antibiotic use through medical benefits and has shown reported effectiveness [28,31].
Since 2006, the Korean government has been publicly reporting the rate of antibiotic use for acute upper respiratory tract infections, leading to substantial changes in their usage patterns for these conditions [32].
The Korean Society for Antimicrobial Therapy has developed smartphone applications to facilitate antibiotic prescriptions. These applications are free of charge and serve as accessible tools for supporting ASPs under such circumstances. Efforts are currently ongoing to further promote and expand the use of these applications [33].
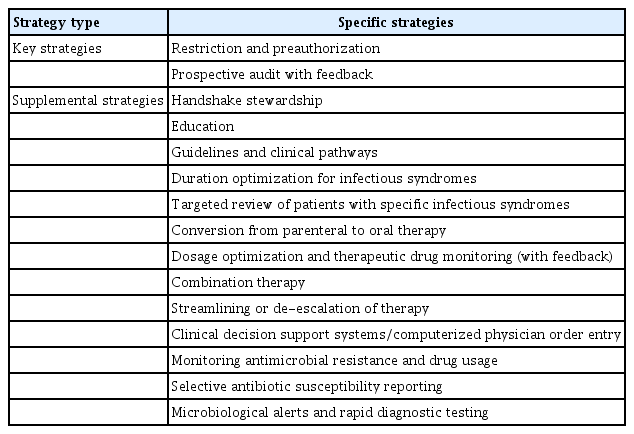
ASPs strive to encourage the correct use of antimicrobials in healthcare settings to address AMR [1]. In 2021, the “Guidelines on Implementing Antimicrobial Stewardship Programs in Korea” were introduced for the first time, highlighting the effectiveness and strategies of ASPs [22]. The core strategies include restrictions and prior authorization for antimicrobial use, prospective audits with feedback, and specific infection syndrome guidelines [22]. Supplemental strategies include education, therapy optimization and transition, dose and therapy rationalization, clinical decision support systems, and AMR and drug use monitoring [22]. The successful implementation of these strategies requires a multidisciplinary approach, which involves collaborative efforts between healthcare providers and ASP teams as well as ongoing monitoring and evaluation to improve antimicrobial use and combat AMR (Table 1) [1,7,22].
The need for collaboration among academia, public agencies, and the industry is critical for improving the effectiveness of ASPs and overcoming the challenge of antibiotic resistance. A collaborative approach is essential for pooling resources, expertise, and innovative strategies to ensure a strong and integrated response to this urgent global health threat. The engagement of all sectors is necessary to advance and implement successful ASPs, which are key to preserving the efficacy of antibiotics for future generations [2,8]. Furthermore, there is an urgent need for the rapid introduction of new antibiotics in Korea to combat emerging resistance.
CORE ELEMENTS OF ASPs IN KOREA
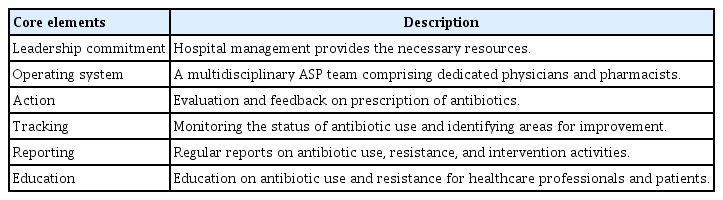
In 2022, for the first time, the “Core Elements for Implementing Antimicrobial Stewardship Programs in Korean General Hospitals” were established through a Delphi consensus procedure by multidisciplinary expert panels [21,34]. This initiative outlines an organizational framework and activities for ASPs specifically tailored to the Korean context (Table 2).
1) Leadership Responsibilities: Hospital leadership is responsible for coordinating ASPs, allocating necessary resources, and prioritizing the implementation of such ASPs. Support from senior management is crucial for the success of the program, and hospitals should leverage governmental incentives.
2) Operational Framework: An interdisciplinary ASP team should be established, which consists of experts such as pharmacists and physicians specializing in antibiotic use. Staff roles must be clearly defined with a focus on antibiotic use management.
3) Interventions: Various interventions, including prescribing assessments and electronic support systems, are essential for optimizing antibiotic use. Core interventions encompass prescription restrictions and treatment guidelines for antibiotic use.
4) Monitoring: Ongoing assessments should be performed to evaluate antibiotic use and the effectiveness of ASP. Furthermore, monitoring protocols should include surveillance of antibiotic usage, MDR bacteria, and adherence to restrictions in prescription.
5) Reporting: Periodic reports on antibiotic use and resistance should be submitted to hospital management and shared with relevant staff. These reports will provide information on ongoing strategies and interventions.
6) Education: Continuous educational initiatives should target healthcare professionals, hospital staff, patients, and caregivers to promote proper antibiotic usage and stewardship.
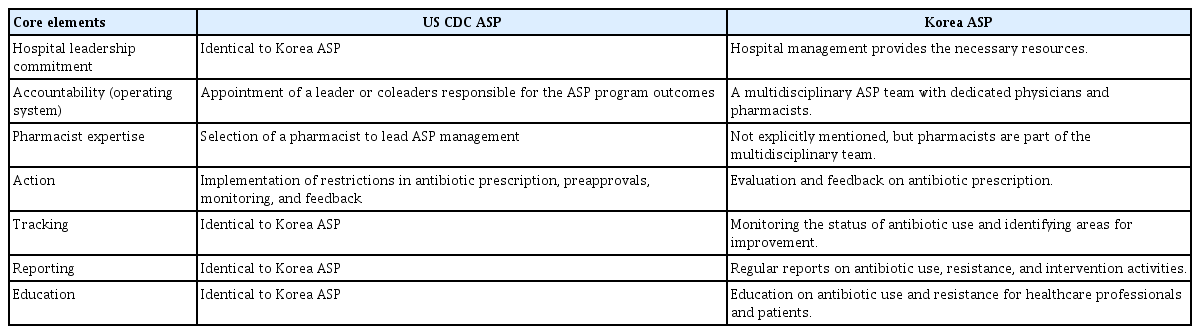
As presented in Table 3, ASPs in the USA and Korea share several core elements, including an emphasis on leadership commitment, antibiotic use monitoring, periodic reporting, and educational initiatives targeting healthcare providers and patients [1,7,21,35]. Despite these similarities, notable differences remain. In the USA, accountability for ASP outcomes rests with a designated leader or coleaders, whereas in Korea, this responsibility is collectively shared by a multidisciplinary team that includes physicians and pharmacists [1,7,21,35]. Additionally, the USA model explicitly mandates a pharmacist to assume a leadership role in ASP management, a stipulation not present in the Korean model, where pharmacists are team members but not explicitly designated as leaders [1,7,21,35].
Few hospitals in Korea implement the core elements of ASPs [21]. The establishment of ASPs in Korean hospitals requires substantial support from both the government and hospital leadership. Each hospital requires well-trained staff to effectively implement ASPs. However, Korea faces challenges due to a shortage of trained infectious disease specialists and pharmacists to lead these programs. To overcome this issue, a comprehensive training program to develop professionals in ASP management is necessary. Additionally, healthcare facilities require financial support to initiate and sustain ASPs, which could include funding from the National Health Insurance for ASP-related activities.
MULTIDISCIPLINARY TEAM COMPOSITION AND ACTIVITIES FOR ASPs IN KOREA
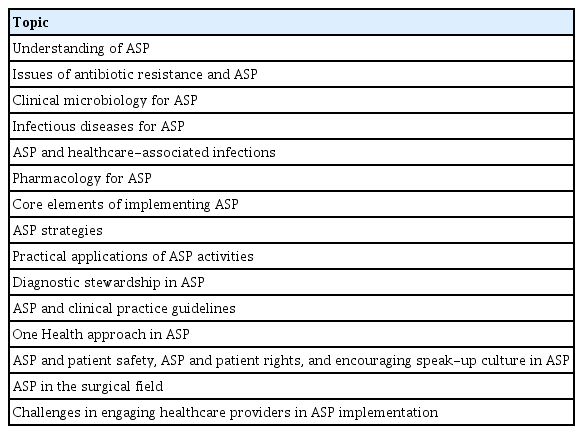
The ASP team should include infectious disease specialists, pharmacists, clinical microbiologists, infection control experts, nurses, and information technology experts. Each member plays a specific role, ranging from spearheading ASP initiatives to providing specialized expertise (Fig. 1) [7,21,22]. While some healthcare institutions in Korea have independently established and operated their own ASP teams, most lack the manpower and resources necessary for such teams. Consequently, systemic and financial support is warranted. National preparations are underway to create a structured training system for ASP experts. The Korean Society for Antimicrobial Therapy has outlined 15 essential educational topics for team members involved in ASPs (Table 4). In addition, plans are in place to facilitate the institutional rollout of ASP teams through both initial and ongoing educational programs. Trained individuals are expected to maintain their practical competencies through active participation in ASP teams at their respective healthcare institutions.
THE ONE HEALTH APPROACH AND ASPs
The widespread use of antibiotics in human, environmental, and animal domains, including livestock and aquaculture, necessitates the One Health approach to synchronize antibiotic management across such domains [36]. Global health organizations such as the WHO emphasize the need for a multifaceted strategy to address antibiotic resistance [37]. This strategy includes enhanced surveillance, data collection, and investment in the research and development of new antibiotics, vaccines, and diagnostic tools. In line with this, the WHO recommends discontinuation of routine use of antibiotics for growth promotion and disease prevention in animals to preserve their efficacy [37]. This recommendation is part of a broader, international, and consumer-driven movement aimed at minimizing antibiotic use in livestock, with proposed alternatives including improved hygiene, vaccinations, and better farming practices. Notably, the European Union banned the use of antibiotics for growth promotion in 2006 [38].
The emergence of antimicrobial-resistant bacteria in poultry meat is considered a public health concern. In Korea, a certification mark titled “Antibiotic-Free Animal Products” promotes antibiotic-free alternatives in animal agriculture, marking progress toward integrated antibiotic management practices across various sectors [39]. However, a recent study demonstrated that both organic or antibiotic-free and conventional chicken products are equally likely to be contaminated with MDR Escherichia coli, highlighting the need for vigilant surveillance and stricter control over antibiotic use in animals [39].
The concept of the antibiotic resistome, which encompasses all antibiotic resistance genes (ARGs), has spurred active research across various One Health sectors for over a decade. This approach is crucial for understanding ARG transmission among humans, animals, and the environment. Advanced methodologies are needed to unravel the complex structure of the resistome [40]. Thus, promoting the One Health approach to ASP in Korea is essential.
Furthermore, the recent documentation of antibiotic resistance in pets and its subsequent transmission to humans in Korea warrants close attention [41].
THE SPEAK UP, GET SMART CAMPAIGN
In the realm of infectious diseases, prudent use of antibiotics is paramount to prevent and combat antibiotic resistance. The “Speak Up, Get Smart” campaign encourages patients to play an active role in their healthcare decisions, particularly concerning antibiotic use [1,42,43]. Awareness regarding the necessity and proper dosage of antibiotics enables the patients to contribute to the judicious usage of antibiotics. This campaign also emphasizes that healthcare providers should cautiously prescribe antibiotics, tailoring them to the individual conditions of patients to effectively combat antibiotic resistance [16]. In addition to educating both patients and providers, the campaign aims to raise public awareness regarding the risks associated with antibiotic resistance and the need to develop new antibiotics to ensure long-term effectiveness [43].
In Korea, a campaign with the message “Antibiotics are not cold medicine” has been initiated. Beyond such initiatives, there is a need for educational resources aimed at the public. These resources could include various promotional materials, fact sheets, educational slides, and brochures disseminated through diverse platforms such as online media, YouTube, and in-person events. Such educational efforts are designed to raise patient awareness about antibiotic resistance and promote the appropriate use of antibiotics.
INTERNATIONAL COOPERATION IN COMBATING AMR
Korea has proactively engaged in combating AMR both nationally and internationally. Following the 2015 WHO global action plan on AMR, the country established the Kor-GLASS in 2016 [26,44]. This system plays a crucial role in monitoring drug-resistant pathogens and providing essential data to effectively manage AMR. Reports from Kor-GLASS have indicated considerable AMRs among various bacteria, highlighting the need for improved hospital infection control and hospitalization systems in Korea [26,45].
Moreover, Korea has been an active participant in international efforts to combat AMR [46]. The country joined the Global Antimicrobial Resistance Surveillance System led by the WHO and is involved in the creation of database for AMR in animals, organized by the OIE-World Organization for Animal Health [47].
FUTURE DIRECTIONS FOR ASP IN KOREA
Establishing comprehensive systems and providing specialized training for personnel involved in ASPs are essential for the continued progress of Korea in this field. These initiatives represent key steps toward enhancing the effectiveness of ASPs and combating antibiotic resistance in the country.
To further this effort, prioritizing strategies to reduce the duration of antibiotic usage is imperative. Decreasing overall antibiotic use is crucial because the quantity of antibiotics used substantially contributes to the development of resistance [48,49]. Additionally, incorporating outpatient parenteral antibiotic therapy (OPAT) into ASP activities is of great importance. OPAT can effectively reduce hospital stays and healthcare costs, thereby improving patient outcomes and resource allocation [50,51]. These targeted strategies, alongside the foundational work of system establishment and training, are critical for enhancing the effectiveness and impact of ASPs in Korea.
CONCLUSION
This review emphasizes the escalating challenge of antibiotic resistance and its considerable impact on global health, highlighting the need for ASP implementation. It outlines the importance of multidisciplinary collaboration, guided by specific Korean guidelines and core elements. Additionally, it highlights the instrumental role of patient-centric campaigns in addressing antibiotic resistance. The review also points out the challenges faced in Korea, including shortages in resources, systems, and personnel, and how these impede ASP efforts. To overcome these challenges, it emphasizes the need for effective policies and the establishment of a robust reward system to encourage and sustain best practices in antibiotic use.
Notes
CRedit authorship contributions
Ki Tae Kwon: conceptualization, writing - original draft, writing - review & editing; Shin Woo Kim: conceptualization, writing - original draft, writing - review & editing, visualization, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None