Risk factors for progressing to critical illness in patients with hospital-acquired COVID-19
Article information
Abstract
Background/Aims
Risk factors for progression to critical illness in hospital-acquired coronavirus disease 2019 (COVID-19) remain unknown. Here, we assessed the incidence and risk factors for progression to critical illness and determined their effects on clinical outcomes in patients with hospital-acquired COVID-19.
Methods
This retrospective cohort study analyzed patients admitted to the tertiary hospital between January 2020 and June 2022 with confirmed hospital-acquired COVID-19. The primary outcome was the progression to critical illness of hospital- acquired COVID-19. Patients were stratified into high-, intermediate-, or low-risk groups by the number of risk factors for progression to critical illness.
Results
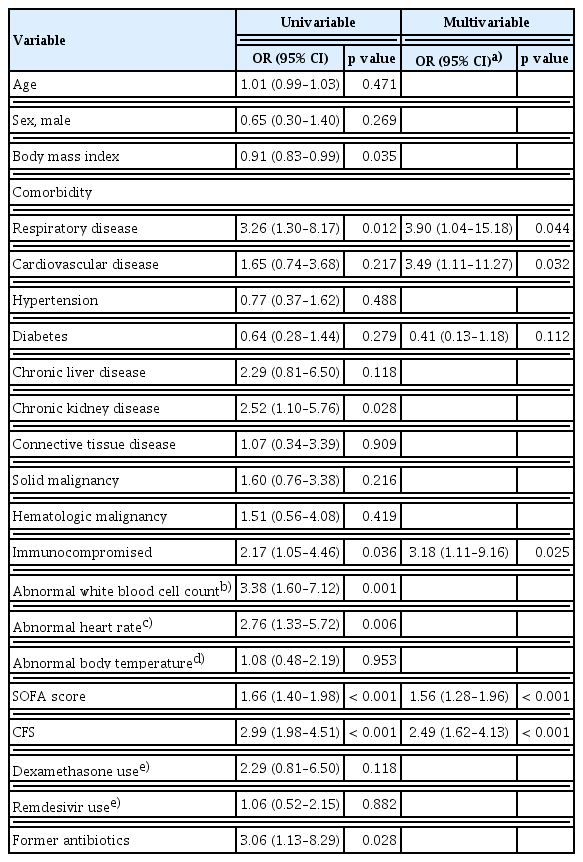
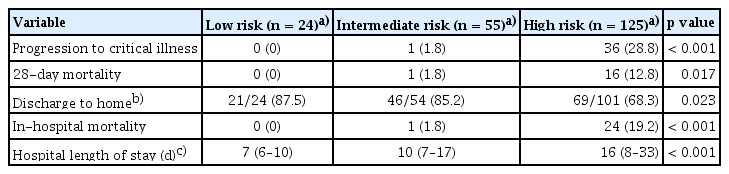
In total, 204 patients were included and 37 (18.1%) progressed to critical illness. In the multivariable logistic analysis, patients with preexisting respiratory disease (OR, 3.90; 95% CI, 1.04–15.18), preexisting cardiovascular disease (OR, 3.49; 95% CI, 1.11–11.27), immunocompromised status (OR, 3.18; 95% CI, 1.11–9.16), higher sequential organ failure assessment (SOFA) score (OR, 1.56; 95% CI, 1.28–1.96), and higher clinical frailty scale (OR, 2.49; 95% CI, 1.62–4.13) showed significantly increased risk of progression to critical illness. As the risk of the groups increased, patients were significantly more likely to progress to critical illness and had higher 28-day mortality.
Conclusions
Among patients with hospital-acquired COVID-19, preexisting respiratory disease, preexisting cardiovascular disease, immunocompromised status, and higher clinical frailty scale and SOFA scores at baseline were risk factors for progression to critical illness. Patients with these risk factors must be prioritized and appropriately isolated or treated in a timely manner, especially in pandemic settings.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) began in 2019 and continues to be a global pandemic, causing over 700 million infections and over 6 million deaths by 2022. The use of vaccines against SARS-Cov-2 infection has helped reduce the risk of transmission and the severity of the disease [1]. Moreover, treatments, such as dexamethasone, baricitinib, tocilizumab, and remdesivir have been effective in reducing mortality rates and shortening treatment periods [2]. Consequently, social distancing and hospital transmission prevention measures have been relaxed. Despite this, the risk of COVID-19 remains high as the pandemic continues to result in new infections and deaths worldwide.
COVID-19 can range from a mild illness with few or no symptoms to a critical illness that progresses to respiratory failure, shock, and multiple organ failure [3]. Several studies have identified risk factors for the progression to critical illness in patients with COVID-19, such as age, male sex, obesity, chronic heart disease, chronic respiratory disease, diabetes, chronic kidney disease, cancer, fever, D-dimer elevation, lymphopenia, neutrophilia, and troponin elevation [4–8]. Based on these studies, risk-scoring systems have been developed and validated to predict progression to critical illness in patients with COVID-19 disease [9,10]. It has been shown that the mortality rate is higher in critical cases than in mild and severe cases [3].
In hospital settings, SARS-CoV-2 can easily spread through various routes, such as person-to-person transmission or from the surface of a contaminated object to a person [11]. Moreover, many patients hospitalized for illnesses other than COVID-19 already have several risk factors for progression to critical illness in community-acquired COVID-19. However, few studies have been conducted on the risk factors for progression to critical illness in patients with hospital-acquired COVID-19. Moreover, in the event of an in-hospital viral outbreak, it may not be feasible to isolate or treat all hospitalized patients in a timely manner, especially in pandemic settings. This creates difficulties in determining which patients should prioritize isolation and prevention and in managing the transmission and spread of viral infections [12] and resource-utilization decisions.
In this study, we hypothesized that patients with progression to critical illness in hospital-acquired COVID-19 are at an increased risk of worse clinical outcomes. Here, we assessed the incidence and risk factors for progression to critical illness and determined their effects on clinical outcomes in patients with hospital-acquired COVID-19.
METHODS
Study design and patients
We conducted a retrospective cohort study of patients admitted to Seoul National University Hospital, a 1,778-bed tertiary academic hospital in South Korea, between January 1, 2020 and June 30, 2022. We included patients aged 19 years who had confirmed hospital-acquired COVID-19 in a general ward. In accordance with domestic guidelines during the COVID-19 outbreak, patients were admitted to the hospital after a confirmed negative SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) result. Hospital-acquired COVID-19 was defined as a positive SARS-CoV-2 RT-PCR result confirmed 5 days after hospitalization. Considering the incubation period of SARS-CoV-2 infection [13], patients diagnosed with COVID-19 within 5 days of hospitalization were classified as community-acquired and ineligible for the study. The SARS-CoV-2 RT-PCR result was considered positive at a cycle threshold of less than 36. Patients were excluded if they were undergoing noninvasive or mechanical ventilation before the diagnosis of hospital-acquired COVID-19 or were discharged within 24 h of the diagnosis of hospital-acquired COVID-19. This study was conducted in accordance with the principles of the Declaration of Helsinki. The Institutional Review Board of Seoul National University Hospital waived the requirement for written informed consent owing to the retrospective nature and approved this study (No. IRB-H-2207-164-1344).
Data collection
We collected and analyzed data on baseline and clinical characteristics, treatments related to COVID-19, and clinical outcomes. Baseline characteristics were investigated at the time of the diagnosis of hospital-acquired COVID-19 in the general ward. Based on the definitions used in previous studies, immunodeficiency was defined as any of the following conditions within the last 90 days: chemotherapy; immunosuppressants, including corticosteroids (prednisolone ≥ 20 mg/day, or an equivalent dose of other corticosteroids, for 2 weeks or longer); human immunodeficiency virus infection or acquired immune deficiency syndrome; or immunosuppressive disease, such as hypogammaglobulinemia [14–16]. For preexisting respiratory disease, comorbidities such as chronic obstructive pulmonary disease, interstitial lung disease, non-tuberculous mycobacterial pulmonary disease, pulmonary tuberculosis, and post-tuberculosis-related lung disease were included. Preexisting cardiovascular disease comorbidities, such as heart failure, ischemic heart disease, arrhythmia, peripheral vascular disease, abdominal aorta aneurysm, pericarditis, and cardiac valve disease were included. Patients with clinical frailty scale (CFS) score ≥5 were classified as “frail” [17].
The clinical characteristics of patients with COVID-19 disease were investigated, including their need for respiratory support, such as conventional oxygen therapy (e.g., nasal prong and face mask), high-flow nasal cannula (HFNC), non-invasive ventilation, and mechanical ventilation. We also assessed the patients’ condition at discharge (discharge to home, nursing home, or transfer to another hospital), in-hospital mortality, and 28-day mortality.
Study outcomes
The primary outcome was progression of hospital-acquired COVID-19 to a critical illness. Based on the World Health Organization (WHO) living guideline and Centers for Disease Control and Prevention COVID-19 guidance [2,18], critical illness was defined as any of the following conditions within 4 weeks after the diagnosis of hospital-acquired COVID-19: acute respiratory distress syndrome (ARDS), septic shock, or the need for life-sustaining therapy (vasopressor, noninvasive ventilation, HFNC, or mechanical ventilation). ARDS was defined according to the Berlin criteria [19] and septic shock was defined as the use of vasopressors and a serum lactate level exceeding 2 mmol/L in patients with sepsis [20]. Patients receiving HFNC prior to hospital-acquired COVID-19 diagnosis were considered critically ill if they progressed to ARDS or septic shock within 4 weeks after the diagnosis of hospital-acquired COVID-19. Secondary outcomes were 28-day mortality, in-hospital mortality, discharged to home, and length of hospital stay.
Statistical analysis
Continuous variables are reported as mean (standard deviation) or median (interquartile range), and categorical variables are reported as counts and percentages. Between-group differences in baseline characteristics were assessed using Student’s t-test or the Mann–Whitney U test for quantitative variables and the chi-square test or Fisher’s exact test for qualitative variables.
Univariable and multivariable logistic regression analyses were used to identify risk factors for progression to critical illness in patients with hospital-acquired COVID-19. Independent variables were selected based on biological plausibility and associations in the scientific literature. We used a bidirectional stepwise regression approach with a significance level of entry (SLE) and a significance level of stay (SLS); both set at 0.15. This approach involved performing multivariate logistic regression on all covariates, iteratively removing variables that did not meet the SLS criterion of 0.15 and adding variables that met the SLE criterion of 0.15, to determine the final model [21]. We generated a receiver operating characteristic (ROC) curve and estimated the area under the receiver operating characteristic (AUROC) curve to determine the predictive value and optimal cut-off values of significant variables identified in the multivariable logistic regression analysis of the risk factors for progression to critical illness in patients with hospital-acquired COVID-19. The optimal cutoff values were determined based on Youden’s index, which maximizes the sum of sensitivity and specificity. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Patients were stratified into high-, intermediate-, and low-risk groups according to the number of risk factors for progression to critical illness in patients with hospital-acquired COVID-19. For continuous variables, the Kruskal-Wallis test was performed to compare the outcomes between the groups. For categorical variables, the chi-square test was used to compare the outcomes between the groups. Statistical significance was defined as a two-sided p value of < 0.05. All statistical analyses were performed using R version 4.1.3. (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).
RESULTS
Baseline characteristics
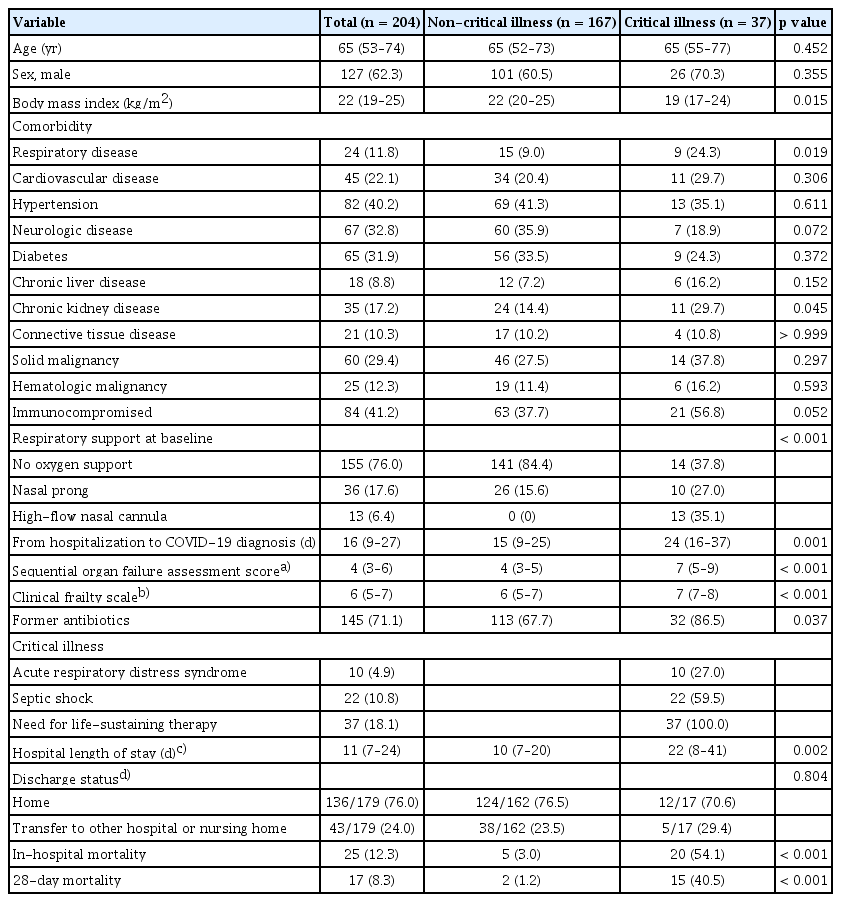
A total of 229 adult patients were confirmed to have hospital-acquired COVID-19 between January 1, 2020 and June 30, 2022. After excluding 16 patients who were discharged on the day of hospital-acquired COVID-19 diagnosis and 9 patients who were receiving non-invasive ventilation or mechanical ventilation prior to hospital-acquired COVID-19 diagnosis, 204 patients were included in this study (Fig. 1). The median age was 65 years (53–74 yr) and 127 (62.3%) patients were male. Of 204 patients, 37 (18.1%) progressed to critical illness (Table 1). The critical illness group had a lower body mass index (BMI) (19 [17–24] kg/m2 vs. 22 [20–25] kg/m2; p = 0.015), more preexisting respiratory diseases (24.3% vs. 9.0%; p = 0.019), and a longer time from hospitalization to diagnosis of COVID-19 (24 [16–37] days vs. 15 [9–25] days; p = 0.001) than the non-critical illness group. The critical illness group had lower oxygen saturation/fraction of inspired oxygen ratio (339 [194–452] mmHg vs. 457 [448–462] mmHg; p < 0.001), higher Pandemic Respiratory Infection Emergency System Triage (PRIEST) COVID-19 clinical severity score (13 [11–14] vs. 8 [6–10]; p < 0.001), Coronavirus clinical characterization consortium (4C) mortality score (10.6 ± 3.7 vs. 8.0 ± 3.3; p < 0.001), sequential organ failure assessment (SOFA) score (7 [5–9] vs. 4 [3–5]; p < 0.001), and CFS (7 [7–8] vs. 6 [5–7]; p < 0.001) than the non-critical illness group at baseline (Table 1, Supplementary Table 1). The incidence of progression to critical illness increased with higher SOFA and CFS scores (Fig. 2).
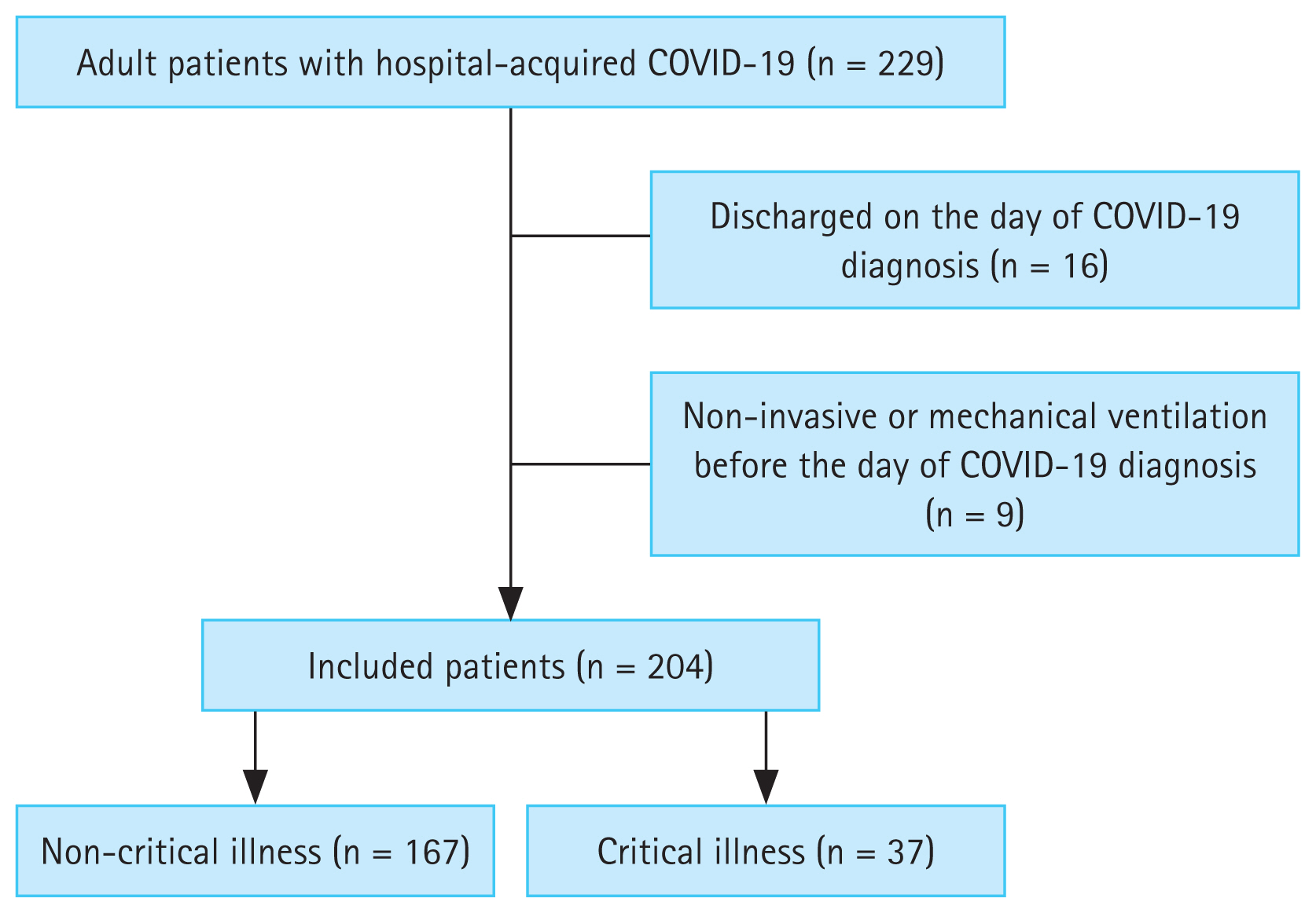
Flowchart of patients. Hospital-acquired COVID-19 was defined as a positive SARS-CoV-2 RT-PCR result confirmed 5 days after hospitalization. Patients were excluded if they were undergoing noninvasive or mechanical ventilation before the diagnosis of hospital-acquired COVID-19 or were discharged within 24 hours of the diagnosis of hospital-acquired COVID-19. COVID-19, coronavirus disease 2019; RT-PCR, reverse transcription-polymerase chain reaction.
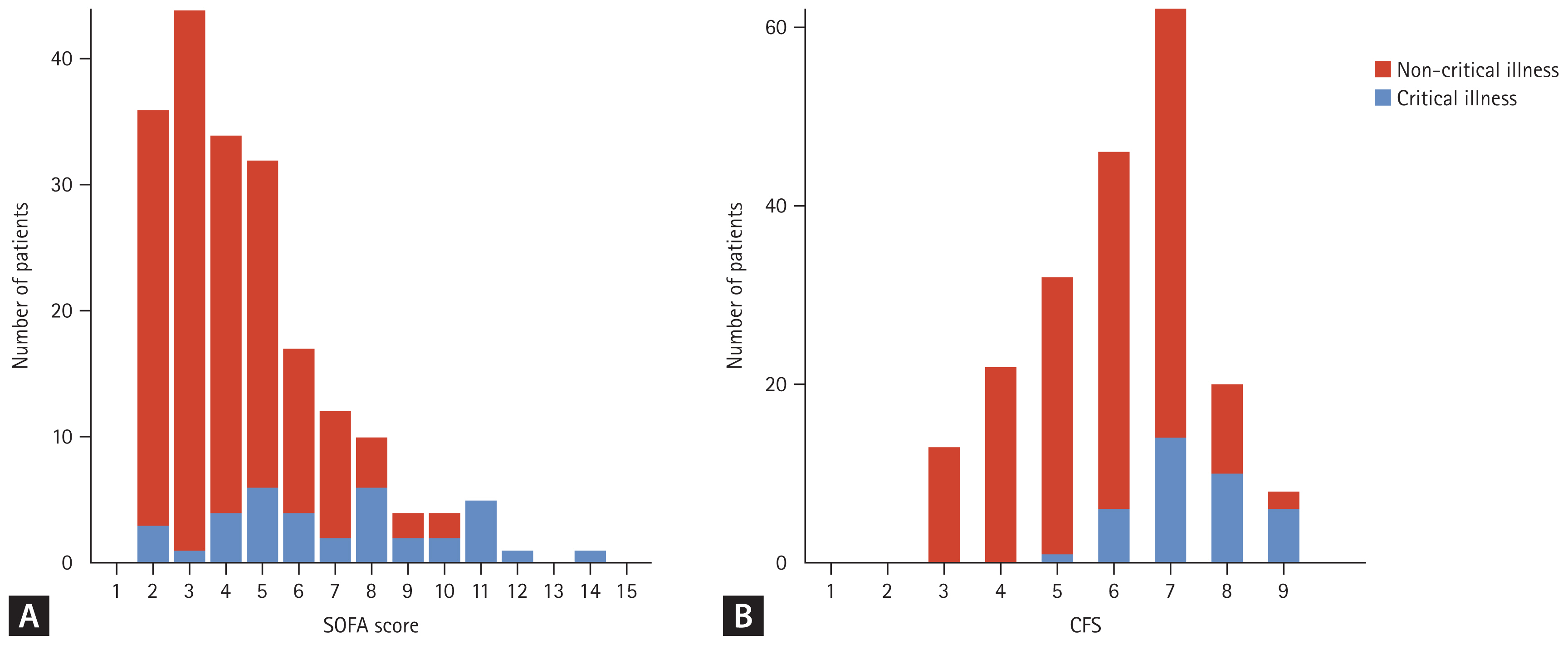
The number of patients with critical and non-critical illnesses according to the baseline characteristics. For each SOFA score (A) and CFS (B), the number of patients with critical and non-critical illnesses is shown. SOFA, sequential organ failure assessment; CFS, clinical frailty scale.
The critical illness group had a longer hospital stay after COVID-19 diagnosis (22 [8–41] days vs. 10 [7–20] days; p = 0.002), higher in-hospital mortality (54.1% vs. 3.0%; p < 0.001), and higher 28-day mortality (40.5% vs. 1.2%; p < 0.001) (Table 1). Median time from COVID-19 diagnosis to critical illness was 4 (0–10) days. Of the two non-critical patients who died within 28 days, one died from gastrointestinal bleeding due to esophageal varices and the other died from cancer progression.
Treatments related to COVID-19
The use of antibiotics (83.3% vs. 58.7%; p = 0.010), dexamethasone (40.5% vs. 7.6%; p < 0.001), and remdesivir (81.1% vs. 47.3%; p < 0.001) within 2 weeks of COVID-19 diagnosis was significantly higher in the critical illness group than in the noncritical illness group (Supplementary Table 2). Two non-critical patients received vasopressors: one had hypovolemic shock due to diuretic use, and the other had atrial fibrillation with a rapid ventricular response and low blood pressure that improved after cardioversion.
Risk factors for progression to critical illness
In the multivariable logistic analysis, patients with a preexisting respiratory disease (OR, 3.90; 95% CI, 1.04–15.18), preexisting cardiovascular disease (OR, 3.49; 95% CI, 1.11–11.27), immunocompromised status (OR, 3.18; 95% CI, 1.11–9.16), higher SOFA score (OR, 1.56; 95% CI, 1.28–1.96), and higher CFS (OR, 2.49; 95% CI, 1.62–4.13) showed significantly increased risk of progression to critical illness (Table 2). We generated a ROC curve and estimated the AUROC to determine the predictive and optimal cutoff values of the SOFA score and CFS. Using a cut-off value of 4.5, the SOFA score predicted progression to critical illness with an AUROC of 0.79 (95% CI, 0.71–0.88). Using a cut-off value of 6.5, the CFS predicted progression to critical illness with an AUROC of 0.81 (95% CI, 0.74–0.88) (Supplementary Table 3). In the multivariable logistic analysis, using the SOFA score divided into organ-specific scores, patients with higher CFS (OR, 3.24; 95% CI, 1.95–5.99), immunocompromised status (OR, 4.14; 95% CI, 1.43–13.31), preexisting cardiovascular disease (OR, 5.04; 95% CI, 1.49–18.30), and higher pulmonary (OR, 3.57; 95% CI, 1.94–7.35) and cardiovascular (OR, 2.05; 95% CI, 1.38–3.21) SOFA subscores showed significantly increased risk of progression to critical illness (Supplementary Table 4).
The risk stratified groups and clinical outcomes
Further analysis was performed using risk-stratified groups as follows: a high-risk group with two or more risk factors (n = 125), an intermediate-risk group with one risk factor (n = 55), and a low-risk group with zero risk factors (n = 24). As the risk of groups increased, the patients were significantly more likely to progression to critical illness (0% vs. 1.8% vs. 28.8%, respectively; p < 0.001), higher 28-day mortality (0% vs. 1.8% vs. 12.8%, respectively; p = 0.017), higher in-hospital mortality (0% vs. 1.8% vs. 19.2%, respectively; p < 0.001), and longer hospital length of stay (7 [6–10] days vs. 10 [7–17] days vs. 16 [8–33] days, respectively; p < 0.001) (Table 3).
DISCUSSION
There are limited studies on the risk factors for progression to critical illness in patients with hospital-acquired COVID-19, making it difficult to prioritize and isolate such patients during an in-hospital COVID-19 outbreak. In the present study, among patients with hospital-acquired COVID-19, preexisting respiratory disease, preexisting cardiovascular disease, immunocompromised status, and higher CFS and SOFA scores at baseline were risk factors for progression to critical illness, including ARDS, septic shock, and the need for life-sustaining therapy. Analysis of the results of the risk-stratified group showed that, as the number of risks increased, critical illness, 28-day mortality, in-hospital mortality, and length of hospital stay increased. This shows that the risk factors are sensitive enough to predict progression to critical illness and poor outcomes in hospital-acquired COVID-19 patients.
In the present study, age, comorbidities (except for preexisting respiratory disease and cardiovascular disease, and immunocompromised state), neutrophilia, and BMI were not significant risk factors for progression to critical illness. These findings are different from those of previous studies on COVID-19, which did not differentiate between hospital- and community-acquired COVID-19 [7,22]. Unlike previous studies that included the general population, our data focused on patients with hospital-acquired COVID-19 in a tertiary hospital. This reflects the fact that our study population consisted of more vulnerable patients than those in previous studies. The average age of the study participants was older (mean age, 65 yr) and had more comorbidities, such as cardiovascular disease (22.1%), connective tissue disease (10.3%), hematologic malignancy (12.3%), solid malignancy (29.4%), and immunocompromised state (41.2%) than the general population [23]. The in-hospital mortality rate of our study was 12.3%, which was much higher than South Korea’s COVID-19 mortality rate of 0.11%. Accordingly, different baseline characteristics, such as age and comorbidities (except for pre-existing pulmonary disease and cardiovascular disease, and an immunocompromised state), may not have been identified as significant risk factors [24].
There are some plausible explanations for the risk factors of hospital-acquired COVID-19 severity found in the present study. Patients with chronic lung diseases and compromised lung function, such as chronic obstructive pulmonary disease and interstitial lung disease, are susceptible to acute exacerbations due to respiratory infection [25]. Furthermore, COVID-19 has been shown to exacerbate preexisting cardiovascular disease and induce a hypercoagulable state [4,26]. Our results showed that pulmonary and cardiovascular SOFA subscores were significant risk factors for hospital-acquired COVID-19 severity. These findings indicate a potential role of the lungs and heart in the progression to critical illness in hospital-acquired COVID-19 patients. According to the US Centers for Disease Control and Prevention, patients with COVID-19 who are immunocompromised have higher illness severity than those without [18]. Previous studies have shown that increased frailty is associated with poor outcomes and reduced response to infection and vaccines [27,28], suggesting that clinical frailty may be a risk factor for in-hospital mortality in patients with COVID-19 [29]. Moreover, COVID-19 can cause multiple organ failures. Several studies have demonstrated a relationship between higher SOFA scores and poor prognosis in patients with COVID-19 [6,29]. These findings validate our results; however, further research is required to establish a direct causal relationship between risk factors and the deterioration of patients with hospital-acquired COVID-19.
As presented in Table 3, we developed and validated a risk stratification method. Five risk factors were identified for the progression to critical illness in patients with hospital-acquired COVID-19 and patients were classified into high-, intermediate-, or low-risk groups, based on the number of these risk factors. As the risk of the groups increased, patients were significantly more likely to progress to critical illness and had higher 28-day mortality. Prior risk stratification scores, such as 4C mortality score and the PRIEST COVID-19 clinical severity score, enumerate extensive risk factors, complicating their immediate application in real clinical settings. Furthermore, these previous methods were not specifically targeted at hospital-acquired COVID-19. However, we propose a more simplified method that enables efficient decision-making during an in-hospital COVID-19 outbreak.
Our findings have important public implications for the early identification of patients at risk of deterioration from hospital-acquired COVID-19, who have different baseline characteristics from those of the general population. Patients at risk of increased severity of hospital-acquired COVID-19 should be screened aggressively. This approach facilitates early identification and implementation of a care plan, including early antiviral therapy [30,31] and timely corticosteroid use [32] for COVID-19, which can reduce the risk of critical illness at an individual level. When an outbreak occurs in a hospital, it is important to prioritize patients and focus on those who are most likely to require preventive measures [33]. This is because not all infected patients will develop critical illness; it is not possible to quarantine all patients in advance, and there is a need to protect healthcare providers. This is especially true in countries with overcrowded healthcare facilities, lack of personal protective equipment, and lack of proper isolation room facilities, as experienced during the Middle East respiratory syndrome coronavirus outbreaks [34] and early COVID-19 pandemic period [35]. Likewise, appropriately prioritizing, and isolating patients who are at a high risk of progressing to critical illness during the in-hospital COVID-19 outbreak could save resources. As there has been limited research on the risk factors of hospital-acquired COVID-19 deterioration, the results of this study may be valuable in determining which in-hospital patients should receive priority for quarantine or treatment in the event of viral outbreaks, especially in pandemic settings.
This study has several limitations. As this was a retrospective study, several important risk factors for deterioration of COVID-19, such as smoking status, vaccination status, radiologic characteristics, degree of control of comorbidities, SARS-CoV-2 variants, and physician orders for life-sustaining treatment (POLST) issue were not adjusted [4,6,36–38]. Although the specific variants could not be confirmed, all patients except two were diagnosed between January and August 2022, a period characterized by the predominance of the Omicron variant [39]. Moreover, individual patient’s vaccination history is not available in the present study. However, as of January 1, 2022, the second vaccination rate for people aged 18 and older in Korea was 93.3%, and the second vaccination rate for people aged 60 and older was 93% of the population [40]. In the present study, 29 patients had documented POLST during their hospitalization and 17 documented POLST before hospitalization. Among 29 patients who had documented POLST during their hospitalization, 20 progressed to critical illness while 9 did not. In the comparison between groups with and without POLST documentation, there were no significant differences in the COVID-19-related treatments such as remdesivir, dexamethasone, antibiotics, and mechanical ventilation. Our findings indicate that POLST had no impact on the primary outcome, namely the progression to critical illness. This conclusion is supported by the observation that critical illness occurred even in patients with documented POLST. Additionally, our data did not reveal any instances of COVID-19-related mortality among patients with documented POLST who were not confirmed to have critical illness. However, it might have influenced the secondary outcomes of hospital length-of-stay and in-hospital mortality. The management of comorbidities may have a significant effect on the outcomes of this study. It is pertinent to note that the majority of our study participants were actively receiving treatment for their comorbid conditions, following their inclusion after five days of hospitalization. However, there was one notable exception: a patient diagnosed with chronic obstructive pulmonary disease who was not undergoing active treatment for this condition. There is a possibility that in some patients, symptoms may have manifested more than 5 days after infection occurred prior to hospitalization. However, the median duration from hospitalization to COVID-19 diagnosis in our study population was 16 (9–27) days, indicating that most of the patients had a much longer interval than 5 days. Finally, in the present study, a relatively small number of patients were studied, raising the possibility that important variables may not have reached statistical significance.
In conclusion, among patients with hospital-acquired COVID-19, preexisting respiratory disease, preexisting cardiovascular disease, immunocompromised status, and higher CFS and SOFA scores at baseline are risk factors for progression to critical illness. Since it is not possible to quarantine all patients during an in-hospital viral outbreak, patients with these risk factors must be prioritized and appropriately isolated or treated in a timely manner, especially in pandemic settings.
KEY MESSAGE
1. This study adds to the evidence that the incidence and risk factors for progression to critical illness in patients with hospital-acquired COVID-19 are important indicators of clinical outcomes.
2. Preexisting respiratory disease and cardiovascular disease, immunocompromised status, and higher CFS and SOFA scores at baseline are risk factors for progression to critical illness.
3. Patients with these risk factors must be prioritized and appropriately isolated or treated in a timely manner, especially in pandemic settings.
Notes
CRedit authorship contributions
Kyung-Eui Lee: conceptualization, investigation, data curation, formal analysis, writing - original draft, writing - review & editing; Jinwoo Lee: methodology, formal analysis, writing - review & editing; Sang-Min Lee: methodology, formal analysis, writing - review & editing; Hong Yeul Lee: conceptualization, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C1074).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.