Effectiveness of Korean Red Ginseng on fatigue in patients with rheumatic diseases: a randomized, double-blind, placebo-controlled study
Article information
Abstract
Background
To evaluate the effectiveness of Korean Red Ginseng (KRG) in managing fatigue in Korean patients with rheumatic diseases
Methods
Patients were randomly assigned to KRG (2 g/day, n = 60) or placebo (n = 60) groups for 12 weeks of blind phase and then open-label KRG from weeks 12 to 24 (placebo-KRG, continuous-KRG). The primary outcome was the improvement rate in fatigue, defined by an increase in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores at 12 weeks. Secondary outcomes included changes in FACIT-Fatigue and fatigue visual analog scale (VAS) between 0 and 12 weeks and those changes in both indices at 24 weeks.
Results
The study enrolled 120 patients (Sjogren syndrome [n = 53], rheumatoid arthritis [n = 43], or both diseases [n = 24]). The mean age was 50.9 ± 11.6 years, with 97.5% being female. Baseline characteristics were similar between the two groups. The improvement rate in FACIT-Fatigue after 12 weeks was higher in the KRG group than in the placebo group, but the difference was statistically insignificant (38.3% vs. 26.7%, p = 0.242). Improvement in fatigue was observed in both groups by increases in FACIT-F (4.6 vs. 4.0) and reductions in fatigue VAS (-16.0 vs. -12.2) scores at 12 weeks. The most frequently reported adverse events during KRG use were pruritus and urticarial, with no significant difference between the two groups.
Conclusions
Both KRG and placebo groups showed significant reductions in fatigue. KRG treatment for 24 weeks did not reduce fatigue symptoms more than the placebo in patients with rheumatic diseases.
INTRODUCTION
Fatigue is one of the most prevalent symptoms of rheumatic diseases and is reported in up to 80% of patients with rheumatoid arthritis (RA) [1] and approximately 70% of patients with Sjögren’s syndrome (SjS) [2]. Patients with rheumatic disease often complain of disabling fatigue, with no significant change even when the rheumatic disease is controlled [3]. Chronic pain and disease activity are suggested factors related to fatigue in rheumatic diseases [1,4], with several psychosocial contributing factors for fatigue, including anxiety, depression, and sleep disorders [1,2,4]. A pro-inflammatory process could be involved in pain [5], and inflammation could potentially serve as a shared pathway linking fatigue, pain, and depression [4,6]. However, several studies have reported no association between fatigue and circulating levels of cytokines [7,8].
Despite being a prevalent and debilitating symptom in rheumatic diseases, fatigue has not been given adequate attention in clinical practice. Moreover, the optimal treatment for fatigue in patients with rheumatic diseases remains uncertain. Thus, numerous individuals diagnosed with RA have explored the use of complementary and alternative medicine (CAM) in conjunction with conventional treatments [9]. CAM has become increasingly popular for patients with RA, with a reported prevalence of CAM use ranging from 18 to 94% across different regions of the world [10,11]. In Korea, 47.7% of patients with RA received some form of CAM, and 10.5% initiated CAM after RA diagnosis [12]. Ginseng is among the most frequently used single health supplements in Korea [13]. Randomized controlled trials have shown that Korean Red Ginseng (KRG) is effective in improving fatigue in patients with idiopathic chronic fatigue [14], cancer [15], and nonalcoholic fatty liver disease [16]. Furthermore, our previous study [17] suggested that fatigue tended to improve in patients with RA receiving treatment with KRG, although the findings did not reach statistical significance. Therefore, we evaluated the impact of KRG on fatigue in patients with rheumatic diseases.
METHODS
Study design
This double-blind, randomized trial was conducted at Hanyang University Hospital for Rheumatic Diseases in the Republic of Korea. After 12 weeks of a double-blind, placebo-controlled period, all patients received KRG until 24 weeks as the open-label period. Treatment doses were 2 g of KRG using 500 mg tablets manufactured by the Korea Ginseng Corporation (Seoul, Korea). The tablets consisted of ginsenoside Rg1 + Rb1 + Rg3 > 5.5 mg/g and cellulose. Placebo tablets, which were indistinguishable from the KRG tablets in terms of their appearance, weight, color, and flavor, were also supplied by Korea Ginseng Corporation.
Patients were randomly assigned in a 1:1 ratio to receive KRG or placebo for 12 weeks. Randomization was carried out by an independent third party using a computer-generated random sequence. To ensure blinding, both KRG and placebo were administered in identical capsules and boxes, with neither the study investigators nor the participants or their caregivers being aware of the assigned treatment.
The primary outcome was the response rate of interventions defined by improvement in fatigue at week 12 of the double-blind phase, based on the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale. The secondary outcomes were the response rate of interventions assessed by the fatigue visual analog scale (VAS) and the changes in FACIT-Fatigue and fatigue VAS between 0 and 12 weeks. The improvement in fatigue was assessed using VAS and FACIT-Fatigue at week 24 and after 12 weeks of the open-label extension phase.
The baseline assessment for all participants included a comprehensive evaluation that encompassed a physical examination, thorough medical history, and laboratory testing. Outcomes were measured at weeks 12 and 24 (Supplementary Fig. 1). The study protocol was registered with ClinicalTrials.gov (NCT03983408).
Study population
Patients were eligible for inclusion if they: (1) were between the ages of 19–80 years and satisfied either the SjS or RA: SjS classified with 2016 American College of Rheumatology (ACR)/European League of Against Rheumatism (EULAR) criteria or RA classified with 1987 ACR criteria [18] or 2010 ACR/ EULAR criteria [19], (2) complained of persistent fatigue for the past 3 months (fatigue VAS measurement of 50 mm or more).
Exclusion criteria were as follows: (1) pregnancy or breast-feeding, (2) abnormal liver function or kidney function, (3) use of ginseng extract within the last 2 months, (4) known allergy to ginseng extract, (5) regular use of corticosteroids or opioids, (6) comorbid diseases, such as chronic kidney disease, chronic hepatitis, thyroid diseases, cancer, depression, fibromyalgia, or chronic fatigue syndrome, with fatigue as the main symptom.
Ethics statement
All patients provided informed consent. This study was approved by the Institutional Review Board of Hanyang University Hospital (HYUH 2019-01-010).
Outcome measures
Fatigue
We assessed the improvement or worsening of fatigue in FACIT-Fatigue, a scale validated for fatigue assessment in patients with RA [20,21]. The FACIT-Fatigue scale consists of 13 questions scored on a 0–4 Likert scale. This scale yields a summed total score ranging between 0 and 52 (52 = no fatigue). Fatigue was also assessed with VAS (0–100 mm). The improvement is defined as an increase in FACIT-Fatigue score of more than 8.3 [22], and decrease in fatigue VAS of more than 17 based on previous studies [23].
Safety
Safety was reported as the frequency of adverse events (AEs), including clinically significant changes in laboratory test results such as complete blood cell count, liver function tests, and lipid profile. AEs were categorized by a system of organs according to Common Terminology Criteria version 4.03 [24] and classified according to the severity of each AE as mild, moderate, severe, or very severe. Severe AEs (SAEs) were also identified.
Statistical analysis
Continuous data were presented as mean ± standard deviation or median (interquartile range) and compared between KRG and placebo groups by the independent t-test or Mann–Whitney U test, appropriately. Categorical data were presented as frequency (%) by the chi-square test. Efficacy analyses were performed on the intent to treat (ITT) dataset, and multiple imputations by chained equations with the predictive mean matching generated 20 complete datasets [25,26].
For analyzing the primary and secondary outcome, response rates were compared, at 12 and 24 weeks, between KRG and placebo groups based on the contingency table corresponding to the median p value of the chi-square tests in the 20 imputed datasets. In addition, for exclusive patients who successfully completed initially assigned treatment (per protocol [PP] analysis), fatigue indices changes at 12 or 24 weeks from baseline were compared between KRG and placebo groups by the independent t-test. To assess fatigue improvement within each group, we compared fatigue indices at 12 or 24 weeks with respect to baseline scores by the paired t-test. Next, primary and secondary analyses were also performed after dividing whole patients according to underlying diseases: SjS, RA, and both diseases.
The minimal important differences (MID) for fatigue may vary across different populations and contexts. Therefore, as a sensitivity analysis, we applied varying definitions of fatigue improvement based on previous validation studies. Improvement was defined as an increase of either 3 or 4 on the FACIT-Fatigue [20] or a decrease of 6.2 mm or 7 mm on the fatigue VAS [23,27]. Safety analyses were conducted using data obtained from baseline to week 12, baseline to week 24, as well as week 12 to week 24, and were subsequently summarized. Statistical significance was defined as a p value less than 0.05 in a two-tailed test. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
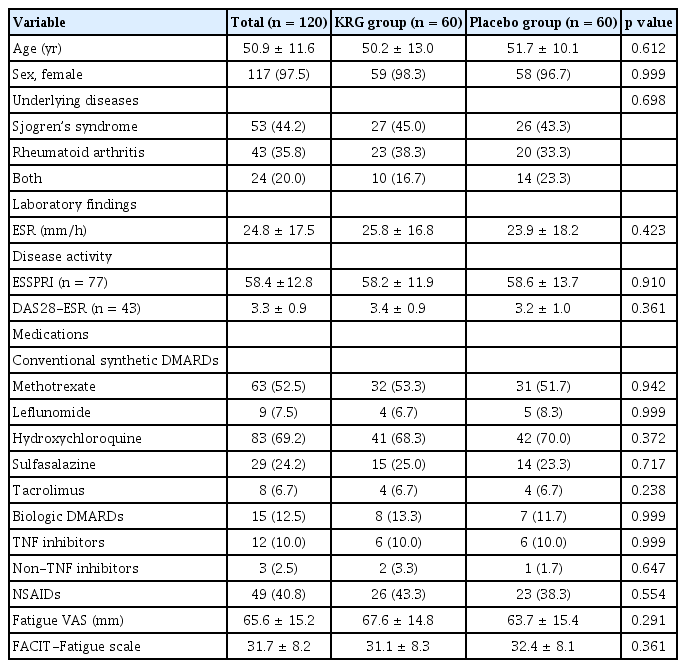
The study initially enrolled 120 patients, and 60 were randomly assigned to the KRG or placebo groups, first receiving either KRG or placebo treatment (Fig. 1). The mean age of patients was 50.9 ± 11.6 years, and 117 were female (97.5%). At baseline, FACIT-Fatigue (31.1 ± 8.3 vs. 32.4 ± 8.1, p = 0.361) and fatigue VAS (67.6 ± 14.8 mm svs. 63.7 ± 15.4 mm, p = 0.291) were comparable between the two groups. There were no differences in baseline characteristics between the two groups (Table 1). In addition, disease activity measured by EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI) or Disease Activity Score of 28 joints-ESR (DAS28-ESR) were not significantly different between treatment groups at baseline, week 12, and week 24 in each disease group (Supplementary Table 1).
Patient withdrawal and follow up
Eight patients withdrew during the double-blind phase; 4 for the KRG group and 4 for the placebo group. In the KRG group, two patients dropped out; one had AE (urticaria), and one discontinued treatment due to protocol deviation (initiation of regular corticosteroid use). In the placebo group, two patients dropped out, one had no follow-up to the hospital, and another dropped out due to an incidentally found lung nodule.
At week 12, 56 patients (93.3%) originally assigned to double-blind KRG transitioned to open-label KRG (continuous KRG group), and 56 patients (93.3%) originally assigned to double-blind placebo transitioned to open-label KRG (placebo-KRG group) until they completed the study or withdrew from treatment. Fifty-five patients in the continuous KRG group and 54 patients in the placebo-KRG group completed week 24. During the open-label extension phase, three patients withdrew: one by request in the continuous KRG group and two patients with arthralgia in the placebo-KRG group (Fig. 1).
Improvement rate of fatigue after the use of KRG
The improvement rates were analyzed under the ITT strategy. In the primary outcome, the improvement rate of fatigue based on the FACIT-Fatigue scale at week 12 of the double-blind phase was numerically higher in the KRG group (38.3%) than in the placebo group (26.7%). However, it was statistically insignificant (p = 0.242) (Fig. 2A). As secondary outcomes, the improvement rate at 24 weeks after interventions of the continuous KRG group decreased from 38.3 to 35.0%, while it increased from 26.7% to 33.3% in the placebo-KRG group after KRG use between 12 and 24 weeks (Fig. 3A).
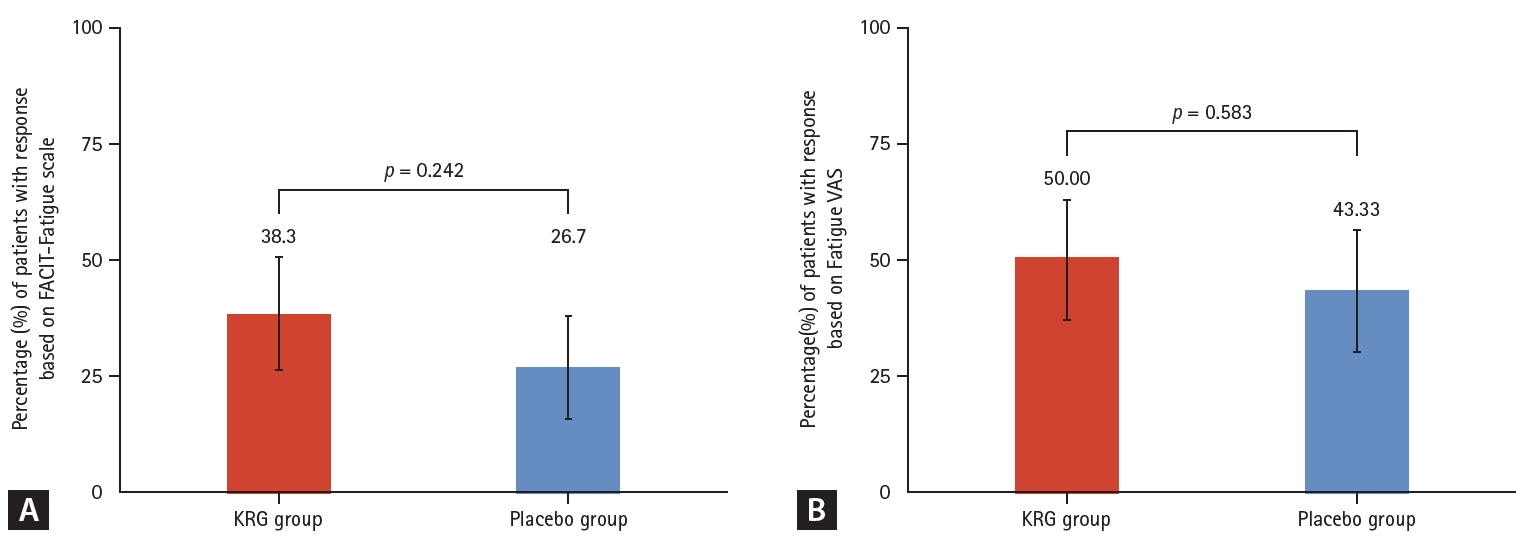
Comparison of response rates during double-blind phase Week 0–12. (A) Response rate based on FACIT-Fatigue scale (change in FACIT-Fatigue scale ≥ 8.3). (B) Response rate based on Fatigue VAS (change in Fatigue VAS ≤ -17). KRG, Korean Red Ginseng; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; VAS, visual analog scale.

Comparison of response rates during the overall period Week 0–24. (A) Response rate based on FACIT-Fatigue scale (change in FACIT-Fatigue scale ≥ 8.3). (B) Response rate based on Fatigue VAS (change in Fatigue VAS ≤ -17). KRG, Korean Red Ginseng; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; VAS, visual analog scale.
The fatigue VAS for assessment of fatigue improvement rate at week 12 was also numerically higher in the KRG group (50.0%) than in the placebo group (43.3%), although it was statistically insignificant (p = 0.583). (Fig. 2B).
At 24 weeks after interventions, the improvement rate of fatigue using patient VAS decreased from 50.0% to 48.3% in the continuous-KRG group, while it increased from 43.3 to 50.0% in the placebo-KRG group (Fig. 3B).
Changes in fatigue indices after the use of KRG
For only those patients who completed the originally allocated treatment, we evaluated the changes in fatigue indices between 0 weeks and 12 weeks or 24 weeks (PP analysis). Over time, fatigue measured using the FACIT-Fatigue scale and Fatigue VAS significantly improved in KRG and placebo groups (Supplementary Fig. 2, Supplementary Table 2). The mean change in FACIT-Fatigue between 0 and12 weeks was also numerically higher in the KRG group (4.6) than in the placebo group (4.0); however, it was statistically insignificant (p = 0.838) (Fig. 4A). Although the mean change in fatigue VAS was numerically higher in the KRG group (-16.0) than in the placebo group (-12.2), there was no statistically significant difference between the two groups (p = 0.438) (Fig. 4B).

Comparison of fatigue improvement between KRG and placebo groups (PP analysis). (A) Fatigue improvement based on FACIT- Fatigue scale during double-blind phase Week 0–12. (B) Fatigue improvement based on Fatigue VAS during double-blind phase Week 0–12. (C) Fatigue improvement based on FACIT-Fatigue scale during the overall period Week 0–24. (D) Fatigue improvement based on Fatigue VAS during the overall period Week 0–24. KRG, Korean Red Ginseng; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; VAS, visual analog scale; PP, per protocol.
When we estimated the mean change of fatigue between 0 and 24 weeks of treatment, FACIT-Fatigue score improvement in the continuous-KRG group was comparable to that in the placebo-KRG group (5.6 vs. 5.6) (Fig. 4C). There was no statistical significance for the change in fatigue VAS; however, the continuous-KRG group exhibited more reduction in fatigue VAS scores than the placebo-KRG group (-16.5 vs. -14.6) (Fig. 4D).
Subgroup analysis
After dividing whole patients into 3 subgroups (SjS, RA, and both diseases), fatigue improvement based on the FACIT-Fatigue scale or fatigue VAS was not significantly different between KRG and placebo groups in each subgroup (Supplementary Fig. 3-5).
Sensitivity analysis
When we changed the set point with the FACIT-Fatigue scale (3 or 4), fatigue VAS (6.2 mm or 7.0 mm) for improvement of fatigue, there were similar response rates between the two groups at week 12 and week 24 (Supplementary Table 3).
Safety
Rates of AEs in the open-label period were higher than those in the double-blind period and were similar between the continuous-KRG and the placebo-KRG group (Supplementary Table 4). Nine patients in the continuous-KRG group experienced an AE during the entire study period: two skin manifestations (pruritus and urticaria), two neoplasms (colon poly and breast mass), each of herpes zoster, arthralgia, ligament rupture on a knee, liver function abnormality, and enteritis. Seven patients in the placebo-KRG group experienced eight AEs during the entire study period: four infections by three patients (lymphadenopathy, herpes zoster, cystitis, and common cold), two arthralgias, one Henoch-Schönlein purpura, and one benign lung nodule.
Three SAEs developed in the continuous-KRG group during the open-label period were one injury, one breast cancer, and one colon polyp. Treatment-related SAEs were absent.
DISCUSSION
This study showed that fatigue significantly improved in both the KRG and placebo groups during 12 weeks of the double-blind period. Although fatigue improvement was better in the KRG group than in the placebo group, there was no statistically significant difference between the two groups.
These results raised several points. First, in the KRG group, fatigue improved markedly during the first 12 weeks of KRG use. In ITT analysis, 38.3% of patients in the KRG group exceeded the cut-off value of improvement of fatigue. Moreover, in PP analysis, fatigue VAS decreased from 67.6 to 51.4 after 12 weeks. Considering that RA and SjS are chronic, systemic inflammatory diseases with a waxing and waning course and feature chronic fatigue, this improvement in fatigue is surprising. However, these results were statistically insignificant because the placebo group also showed significant improvement in fatigue. This finding can be attributed to two factors. First, fatigue is overly subjective and can be influenced by various confounding factors during the assessment. Second, the placebo response of KRG needs discussion. The placebo effect is a psychological and physiological response to placebo treatment; the placebo response is the overall effect of placebo treatment, including both the placebo effect and any other factors that may contribute to the observed outcome. For example, the placebo response may include the natural course of the disease, the effects of other medications, or lifestyle changes that occur during the study period. Moreover, the favorable perception of KRG in Koreans, especially patients enrolled in this clinical trial of KRG, may exhibit more of a placebo response. Participants in this clinical trial who were aware of receiving KRG could have formed positive experiences or perceptions of the efficacy of red ginseng. Placebo responses are often regarded as undesirable interferences to be addressed through trial design and analysis in clinical research [28], or as potential outcomes of participant response bias [29]. However, many previous studies have demonstrated that placebo medications can ameliorate many symptoms, including pain [30] and fatigue [31]. Although placebos are typically known to influence a patient’s perception of symptoms, they may also impact the same physiological systems as active medication, ultimately leading to the modification of physical symptoms [31]. The placebo response is dependent on intricate neurobiological mechanisms that involve neurotransmitters and the activation of specific, measurable, and pertinent brain regions [32]. The genetic signatures of patients likely to respond to placebos are being identified [33]. Therefore, it may be difficult to elucidate the fatigue improvement efficacy of KRG compared to placebos, as placebos can also be an effective intervention for fatigue improvement. After completion of the double-blind phase in our clinical trial, participants were not informed about their assignment to the KRG or placebo groups. This approach may have contributed to the comparable response rates between the two groups. Nevertheless, it is essential to underscore that there is insufficient evidence to assert that the observed fatigue improvement in the KRG group is better than the placebo group.
Fatigue improvement observed over the initial 12 weeks persisted throughout the subsequent 12 weeks of the open-label period of KRG treatment in either the continuous-KRG group or the placebo-KRG group. Our study findings indicate that fatigue reduction observed in the first 12 weeks after intervention surpassed that observed during the subsequent 12–24-week period. Until now, clinical trials have shown the antifatigue effect of KRG in patients with several diseases. Most studies evaluated the short-term efficacy of KRG from 3 weeks to 16 weeks [14-16]. Consequently, KRG efficacy for fatigue improvement may be significant in a relatively short period after the initiation of therapy. Further investigation is warranted to determine whether this effect can be sustained in the long term.
The mechanism by which red ginseng improves fatigue is explained by modulation in cortisol concentrations [34], decreased lactic acid levels in the blood when taking red ginseng, and an increase in the concentration of glutathione peroxidase [35]. However, the biochemical mechanism of KRG for fatigue improvement has not yet been clearly established, and studies on the effect of KRG on fatigue accompanying rheumatic diseases are insignificant. Fatigue in patients with RA is also associated with numerous factors, such as inflammation, pain, disability, and psychosocial factors [36,37]. Among pharmacological treatments known to reduce disease activity in rheumatic diseases, disease-modifying anti-rheumatic drugs such as methotrexate and leflunomide, or biologics (TNF inhibitors, IL-6 inhibitors, CTLA4 immunoglobulin, and anti-CD20) have improved fatigue and pain [4,38].
Most rheumatic diseases are managed with a combination of drugs, such as immunosuppressants, corticosteroids, and anti-inflammatory agents, thus raising concerns about the safety of concomitant use of KRG in clinical practice due to potential drug interactions; therefore, KRG is not routinely recommended in the treatment of rheumatic diseases, which creates challenges for studies on the efficacy of KRG in rheumatic diseases. However, as SjS is an autoimmune disease often accompanied by significant fatigue and typically does not require active administration of immunosuppressants, it may serve as a useful model for studying the effects of KRG on fatigue and immune function in rheumatic diseases.
For safety, the most common AEs of KRG reported were skin manifestations, such as pruritis and urticaria, with no significant difference in frequency and symptoms compared to the placebo group. The symptoms were mild and temporary, with no serious events.
Our study had some limitations. First, we included patients without regular use of corticosteroids or opioids because these drugs could be related to change fatigue. Therefore, most enrolled patients had well-controlled rheumatic disease. In clinical practice, many patients take KRG regardless of disease activity. Moreover, patients often experience more fatigue under high disease activity status. Therefore, since our inclusion criteria restricted patients without corticosteroids, it is difficult to generalize our results for all patients with rheumatic diseases. Based on our study, future studies should enroll patients with a broad range of disease activities to examine the effectiveness of KRG treatment. Second, we did not evaluate changes in blood biomarkers, such as cortisol or cytokine levels. Further investigations are necessary to understand better the mechanisms underlying fatigue improvement observed with KRG. Third, although patient-reported outcome measures are frequently used for fatigue assessment, it is essential to interpret score changes carefully. Our analysis incorporated the MID from previous studies and conducted sensitivity analyses with varying thresholds. However, our study identified inconsistent trends in score changes between the two fatigue assessment tools in some findings. Further investigation will provide deeper insights into these disparities and their clinical significance.
Our study is the first clinical study to provide evidence for the antifatigue properties of KRG in patients with rheumatic diseases. We believe this study will support the development of future clinical trials involving KRG treatment in patients with rheumatic diseases. Next, our study presented relatively long-term efficacy and safety of KRG for 24 weeks in patients with rheumatic diseases.
In conclusion, we report that fatigue significantly improved in both the KRG and placebo groups, with no significant difference between the two groups. KRG intake for 24 weeks was tolerable and safe in patients with rheumatic diseases.
KEY MESSAGE
1. Patients from both the KRG and placebo groups showed notable improvement in fatigue levels as assessed by FACIT-Fatigue scores and fatigue VAS after 12 weeks of treatment. While the improvement rate in fatigue was numerically higher in the KRG group compared to the placebo group after 12 weeks, the difference did not reach statistical significance.
2. The study revealed that KRG treatment over a span of 24 weeks was well-tolerated among patients with rheumatic diseases. AEs during KRG use were primarily pruritus and urticaria, with no significant difference between the KRG and placebo groups.
Notes
CRedit authorship contributions
Soo-Kyung Cho: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - original draft, visualization; Yeo-Jin Song: investigation, data curation, formal analysis, writing - review & editing, visualization; Jung-Yong Han: methodology, investigation, data curation, formal analysis, writing - review & editing, visualization; Hye Won Kim: methodology, resources, formal analysis, writing - review & editing; Eunwoo Nam: methodology, investigation, formal analysis, writing - review & editing, visualization, supervision; Yoon-Kyoung Sung: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - review & editing, visualization, supervision, project administration, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by the 2018 grant from Korean Society of Ginseng.