Clinical impact of pleural fluid carcinoembryonic antigen on therapeutic strategy and efficacy in lung adenocarcinoma patients with malignant pleural effusion
Article information
Abstract
Background/Aims
Epidermal growth factor receptor (EGFR) mutation is important in determining the treatment strategy for advanced lung cancer patients with malignant pleural effusion (MPE). Contrary to serum carcinoembryonic antigen (S-CEA) levels, the associations between pleural fluid CEA (PF-CEA) levels and EGFR mutation status as well as between PF-CEA levels and treatment efficacy have rarely been investigated in lung adenocarcinoma patients with MPE.
Methods
This retrospective study enrolled lung adenocarcinoma patients with MPE and available PF-CEA levels and EGFR mutation results. The patients were categorized based on PF-CEA levels: < 10 ng/mL, 10–100 ng/mL, 100–500 ng/mL, and ≥ 500 ng/mL. The association between PF-CEA levels and EGFR mutation status as well as their therapeutic impact on overall survival was compared among the four groups.
Results
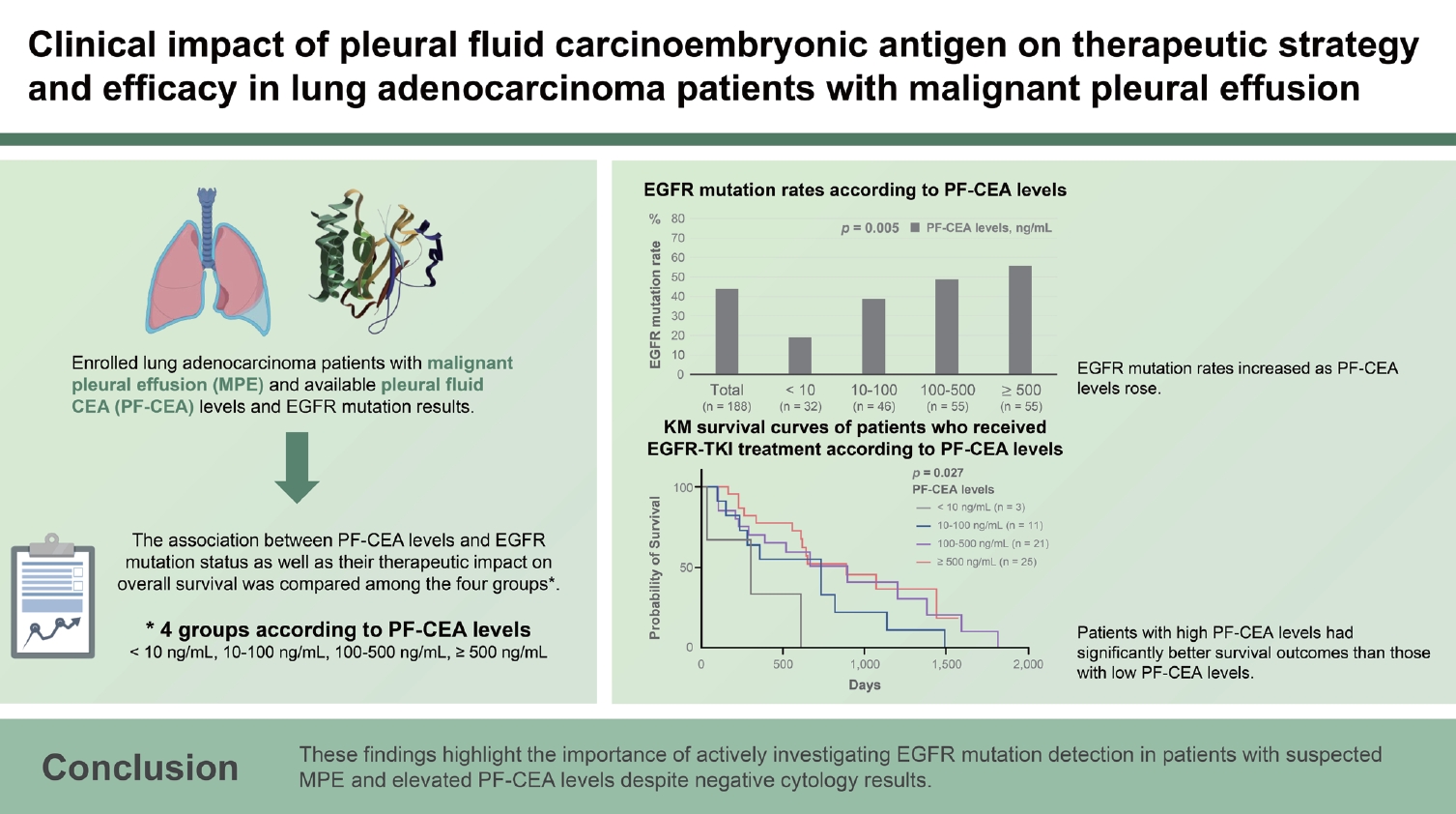
This study included 188 patients. PF-CEA level was found to be an independent predictor of EGFR mutation but not S-CEA level. The EGFR mutation rates were higher as the PF-CEA levels increased, regardless of cytology results or sample types. Among EGFR-mutant lung adenocarcinoma patients receiving EGFR-tyrosine kinase inhibitor (TKI) treatment, those with high PF-CEA levels had significantly better survival outcomes than those with low PF-CEA levels.
Conclusion
High PF-CEA levels were associated with high EGFR mutation rate and may lead to a favorable clinical outcome of EGFR-TKI treatment in EGFR-mutant lung adenocarcinoma patients with MPE. These findings highlight the importance of actively investigating EGFR mutation detection in patients with suspected MPE and elevated PF-CEA levels despite negative cytology results.
INTRODUCTION
Malignant pleural effusion (MPE) is a common complication of advanced non-small cell lung cancer (NSCLC), with lung adenocarcinoma being the main histologic type [1,2]. Epidermal growth factor receptor (EGFR) mutations are most commonly observed in lung adenocarcinoma, and EGFR-tyrosine kinase inhibitor (TKI) treatment has provided superior survival benefits than traditional cytotoxic chemotherapy to patients with advanced lung adenocarcinoma [3,4]. Thus, EGFR mutation detection is important in determining the treatment strategy for lung adenocarcinoma patients with MPE.
Numerous studies have demonstrated that EGFR mutation status in NSCLC is associated with serum carcinoembryonic antigen (S-CEA) levels [5-10]. These studies reported that S-CEA levels could serve as predictors of EGFR mutation status and the frequency of EGFR mutations increases as S-CEA levels rise. However, these results were mainly obtained from NSCLC patients without MPE. Lung cancer patients often experience pleural effusion at initial diagnosis [11]. Pleural fluid CEA (PF-CEA) levels are routinely measured during diagnostic thoracentesis in patients with suspected MPE and have significant clinical implications, suggesting MPE. Similar to S-CEA levels, one study demonstrated that PF-CEA levels could predict EGFR mutation status in patients with MPE [12]. Furthermore, EGFR mutations in lung adenocarcinomas may exhibit discordance between the primary tumor and corresponding metastases [13,14]. Thus, establishing the association between PF-CEA levels and EGFR mutations, using PF-CEA levels instead of S-CEA levels, in lung adenocarcinoma patients with MPE may lead to more precise and closely associated findings related to MPE. However, the association between PF-CEA levels and EGFR mutation status as well as their impact on clinical outcomes has been rarely investigated in advanced lung adenocarcinoma patients with MPE.
This study aimed to investigate the association between PF-CEA levels and EGFR mutations in lung adenocarcinoma patients with MPE and to analyze the impact of PF-CEA levels on EGFR-TKI treatment outcomes of EGFR-mutant lung adenocarcinoma patients with MPE.
METHODS
Patient selection
The medical records of patients at Kyungpook National University Hospital, a tertiary referral hospital in South Korea, were retrospectively reviewed between August 2011 and December 2022. The records of consecutive patients newly diagnosed with MPE were also examined. This study included patients with lung adenocarcinoma who had a confirmed diagnosis of MPE as well as available PF-CEA levels and EGFR mutation results at the time of MPE diagnosis. MPE was confirmed if malignant cells were detected in the pleural fluid or pleural biopsy. The study protocols were approved by the Institutional Review Board of Kyungpook National University Hospital (IRB No. KNUH 2023-05-036). The requirement of informed consent was waived due to the retrospective nature of the study.
Data collection and study design
Data were collected from patients diagnosed with lung adenocarcinoma and MPE, including clinical, laboratory, radiological, cyto-histological, and EGFR mutation findings, obtained at the time of diagnostic thoracentesis. PF- and S-CEA levels, along with other biochemical markers, were simultaneously measured at the time of thoracentesis. The patients were grouped according to PF-CEA levels: < 10 ng/mL, 10–100 ng/mL, 100–500 ng/mL, and ≥ 500 ng/mL. Patient characteristics, EGFR mutation rates, and the therapeutic efficacy of the initial treatment regimen since the MPE diagnosis were compared among the groups based on their respective PF-CEA levels. Overall survival (OS) was calculated from the date of diagnostic thoracentesis for MPE until the date of death.
Detection of EGFR gene mutation and measurement of CEA
EGFR gene mutations spanning exons 18–21 were detected in pleural fluid or biopsy tissue samples using the PNAClampTM EGFR Mutation Detection Kit (PANA-GENE, Inc., Daejeon, Korea) via real-time PCR. PF- and S-CEA levels were measured via chemiluminescence immunoassay.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as median with interquartile range (IQR), and differences between the groups were assessed using Student’s t-test or the Mann–Whitney U test. Categorical variables, on the other hand, were expressed as absolute values and percentages and analyzed using the χ2 test or Fisher’s exact test. Variables with p values of < 0.05 in the univariate analysis were included in multivariate logistic regression analysis to identify independent predictive variables for EGFR mutation. Survival analyses were conducted using the Kaplan–Meier method with a log-rank test. Variables with p values of < 0.05 were considered statistically significant.
RESULTS
Patient characteristics
A total of 443 lung cancer patients with pleural effusion were analyzed in this study. Among them, 223 had lung adenocarcinoma and a confirmed diagnosis of MPE. Of these 223 patients, 29 and 6 did not have available EGFR mutation results and PF-CEA levels, respectively. A total of 188 patients were included in the final analysis, and their characteristics are presented in Table 1. Overall, there were 114 males and 74 females, with a median age of 73 years (IQR, 63–79 yr). Among the patients, 158 were chemotherapy-naïve whereas 30 developed MPE during the previous chemotherapy. Upon stratifying the patients with MPE based on PF-CEA levels, no significant differences were observed between the groups in most clinical, laboratory, and radiographic data. S-CEA levels gradually increased as the PF-CEA levels rose. The result of positive pleural fluid cytology did not significantly differ among the groups, with an overall positive rate of 70%.
EGFR mutation rate
In the overall cohort of 188 patients, 82 (43.6%) had EGFR mutations. The EGFR mutation rate significantly increased as PF-CEA levels rose (p = 0.005), as presented in Table 2 and Figure 1. A similar trend of significant increase was observed in positive cytology samples (p = 0.032) and biopsy tissue samples (p = 0.013). Among the identified mutations, the most common type was the exon 19 deletion (n = 42, 51.2%), followed by the L858R point mutation in exon 21 (n = 35, 42.7%). These mutation distributions did not exhibit significant differences among the four groups.
Factors for predicting EGFR mutation
In both univariate and multivariate analyses conducted to predict EGFR mutation in the overall population, being female sex as well as having high pleural fluid mononuclear leukocytes and elevated PF-CEA levels was identified as an independent predictive factor of EGFR mutation. While S-CEA level was found to be a predictive factor in the univariate analysis, they did not remain significant in the multivariate analysis (Table 3). Furthermore, the PF-CEA to S-CEA ratio was inferior to PF-CEA levels in predicting EGFR mutation in 161 patients with available S-CEA and PF-CEA data (data not shown).
OS according to treatment modalities and PF-CEA levels
After the development and diagnosis of MPE, 60 patients received EGFR-TKI treatment whereas 56 patients received cytotoxic chemotherapy as initial treatment regimens. Best supportive care was provided to 68 patients, whereas anaplastic lymphoma kinase inhibitor was administered to the remaining 4 patients as initial treatment regimen (2 patients with PF-CEA levels of 10–100 ng/mL, 1 patient with levels of 100–500, and 1 patient with levels of ≥ 500 ng/mL). In the Kaplan–Meier survival curves of the 60 EGFR-mutant lung adenocarcinoma patients with MPE who received EGFR-TKI as initial treatment regimen, the group with higher CEA levels had significantly better survival time than those with lower CEA levels (p = 0.027) (Fig. 2A), suggesting that EGFR-TKI treatment is more effective in patients with high CEA levels. Among the 60 patients who received EGFR-TKI therapy, 2 (3.3%) developed MPE during their previous EGFR-TKI therapy and were subsequently treated with third-generation EGFR-TKI due to a newly confirmed EGFR T790M mutation. Although the number of patients in each group was insufficient, most of the clinico-laboratory characteristics of those receiving EGFR-TKI treatment did not show significant differences among the groups (data not shown). Contrarily, among the 56 patients with MPE who received cytotoxic chemotherapy as initial treatment regimen, PF-CEA levels did not affect their OS (Fig. 2B). Seven (12.5%) of them developed MPE during the previous cytotoxic chemotherapy and 6 (10.7%) during the previous EGFR-TKI treatment.
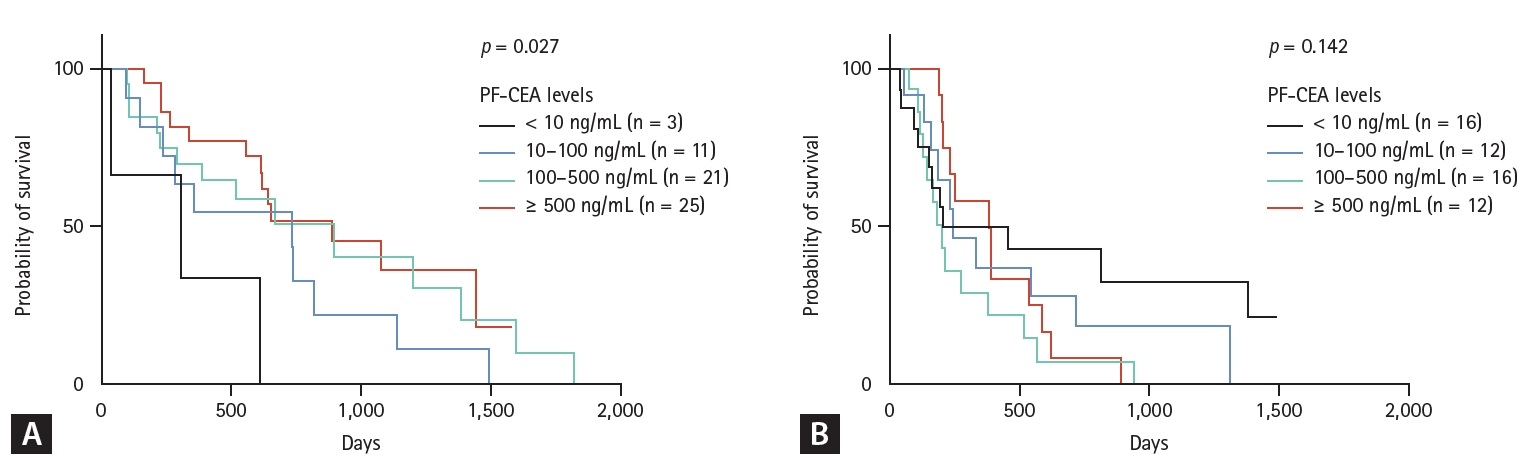
Kaplan–Meier survival curves of patients who received EGFR-TKI Tx (A) and cytotoxic chemotherapy (B) as initial Tx regimen after the diagnosis of malignant pleural effusion according to pleural fluid CEA levels. EGFR-TKI, epidermal growth factor receptor–tyrosine kinase inhibitor; PF-CEA, pleural fluid–carcinoembryonic antigen; Tx, treatment.
DISCUSSION
In this study, the patients were divided into four groups based on PF-CEA levels: < 10 ng/mL, 10–100 ng/mL, 100– 500 ng/mL, and ≥ 500 ng/mL. The main findings were as follows: 1) The EGFR mutation rates were higher as PF-CEA levels increased, regardless of cytology results or sample types. 2) In the multivariate analysis for the prediction of EGFR mutation, PF-CEA level was identified as an independent predictive factor. 3) EGFR-mutant lung adenocarcinoma patients with high PF-CEA levels tended to have more favorable clinical outcomes than those with low PF-CEA levels. These findings suggest that if PF-CEA levels are high in patients with suspected MPE who are undergoing diagnostic thoracentesis, EGFR mutation detection should be actively performed to make an optimal treatment modality decision.
In this study of MPEs secondary to lung adenocarcinoma, the EGFR mutation rates were more strongly associated with PF-CEA levels than with S-CEA levels, consistent with the findings of a previous study [12]. Although S-CEA levels were generally correlated with PF-CEA levels in most patients, some patients with low S-CEA levels had high PF-CEA levels (Supplementary Table 1). Patients with high PF-CEA levels exhibited a relatively high EGFR mutation rate than those with low PF-CEA levels. This discrepancy suggests that PF-CEA level is more reliable than S-CEA level in predicting the EGFR mutation of lung adenocarcinoma patients with MPE.
This study observed a higher EGFR mutation rate with increasing PF-CEA levels. However, this association may not consistently exhibit a positive correlation. For instance, in our cohort, PF-CEA levels of ≥ 1,000 and ≥ 2,000 ng/mL showed EGFR mutation rates of 50.0% (19/38) and 63.2% (12/19), respectively, suggesting a potential plateau phenomenon beyond a certain cutoff value. Further study is warranted to confirm this association.
Positive cytology results were not found to be associated with PF-CEA levels (Table 1) or the rates of positive EGFR mutation (positive cytology [41.6%] vs. negative cytology [48.2%], p = 0.408) (Table 2). The association between PF-CEA levels and EGFR mutation rates exhibited a similar trend in both cytology and biopsy tissue samples. Several recent studies found that supernatant samples of cytology-negative MPE yielded EGFR mutation results compatible with tissue samples [2,15,16]. Furthermore, elevated PF-CEA levels exceeding 40 or 50 ng/mL strongly indicate the presence of MPE, demonstrating a specificity of 100% [17,18]. Taken together, conducting simultaneous EGFR mutation testing on the supernatant sample of pleural fluid, along with cytology, may be beneficial in patients with suspected MPE. The results can be anticipated based on the PF-CEA levels observed during diagnostic thoracentesis. High PF-CEA levels strongly indicate the presence of MPE, particularly adenocarcinoma type with a high EGFR mutation rate. In addition, the EGFR mutation result of the supernatant sample is likely to be consistent with that of the tissue biopsy sample, whether it is positive or negative. These consequent findings can help clinicians determine the most suitable treatment option and decide whether an invasive pleural biopsy is necessary for EGFR mutation assessment.
The expected survival benefit of EGFR-TKI treatment was higher in MPE patients with elevated PF-CEA levels. This observation is consistent with those in previous studies that demonstrated the greater efficacy of EGFR-TKI treatment in patients with elevated S-CEA levels [19,20]. One hypothesis states that a possible antiapoptotic signal of the mutant EGFR pathway may enhance CEA protein expression in tumors [21]. Consequently, patients with elevated PF-CEA levels are more likely to have a higher number of EGFR-mutant tumor cells, potentially increasing the efficacy of EGFR-TKI therapy in these patients. However, this positive effect was limited to patients with positive EGFR mutation. Patients without EGFR mutation did not exhibit difference in OS based on PF-CEA levels. Instead, our study showed that EGFR-mutant-negative lung adenocarcinoma patients with MPE who received cytotoxic chemotherapy might potentially experience unfavorable clinical outcomes if a sufficiently large number of patients were compared (Fig. 2B, p = 0.142). These observed associations between OS and PFCEA levels, based on treatment modalities with and without EGFR mutation, may be attributed to the combined influence of tumor burden and intra-tumor clonal heterogeneity, including the number of tumor cells harboring EGFR mutations [22,23]. However, our study involved a small sample size in each group. Thus, further research that includes a large sample size is warranted to confirm these results and hypotheses.
The present study has several limitations. First, it is a retrospective study conducted at a single center, which inevitably introduces selection bias. Second, the sample size in each group based on CEA levels was relatively small. However, our main findings regarding the association between PFCEA levels and EGFR mutation were supported by a multivariate analysis conducted on 188 individuals. Third, this study did not explore the underlying mechanisms of the association between PF-CEA levels and EGFR mutation, nor did it assess those for the different impacts of PF-CEA levels on clinical outcomes according to treatment modalities. Finally, this study was conducted in a country in East Asia with relatively high EGFR mutation rates. Therefore, these results may differ from those in other ethnic countries.
In conclusion, elevated PF-CEA levels were associated with a higher EGFR mutation rate and could lead to more favorable clinical outcomes in EGFR-mutant lung adenocarcinoma patients with MPE who received EGFR-TKI treatment. These findings suggest that EGFR mutation detection should be actively performed in patients with suspected MPE and high PF-CEA levels despite negative cytology results.
KEY MESSAGE
1. The EGFR mutation rates in lung adenocarcinoma patients with MPE increased as PF-CEA levels rose, and they showed a strong association with PF-CEA levels, which served as a more reliable predictor than S-CEA reported in previous studies.
2. EGFR-mutant lung adenocarcinoma patients with high PF-CEA levels who received EGFR-TKI treatment may have better survival outcomes than those with low PF-CEA levels.
3. Contrarily, high PF-CEA levels may indicate a potential negative prognosis in EGFR wild-type lung adenocarcinoma patients with MPE who received cytotoxic chemotherapy.
Notes
CRedit authorship contributions
Jaehee Lee: conceptualization, data curation, formal analysis, writing - original draft, writing - review & editing; Deok Heon Lee: investigation, data curation, writing - review & editing; Ji Eun Park: investigation, data curation; Yong Hoon Lee: investigation, data curation; Sun Ha Choi: investigation, data curation; Hyewon Seo: investigation, data curation; Seung Soo Yoo: investigation, data curation; Shin Yup Lee: investigation, data curation; Seung-Ick Cha: investigation, data curation; Jae Yong Park: investigation, data curation; Chang Ho Kim: conceptualization, methodology, data curation, writing - original draft, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None