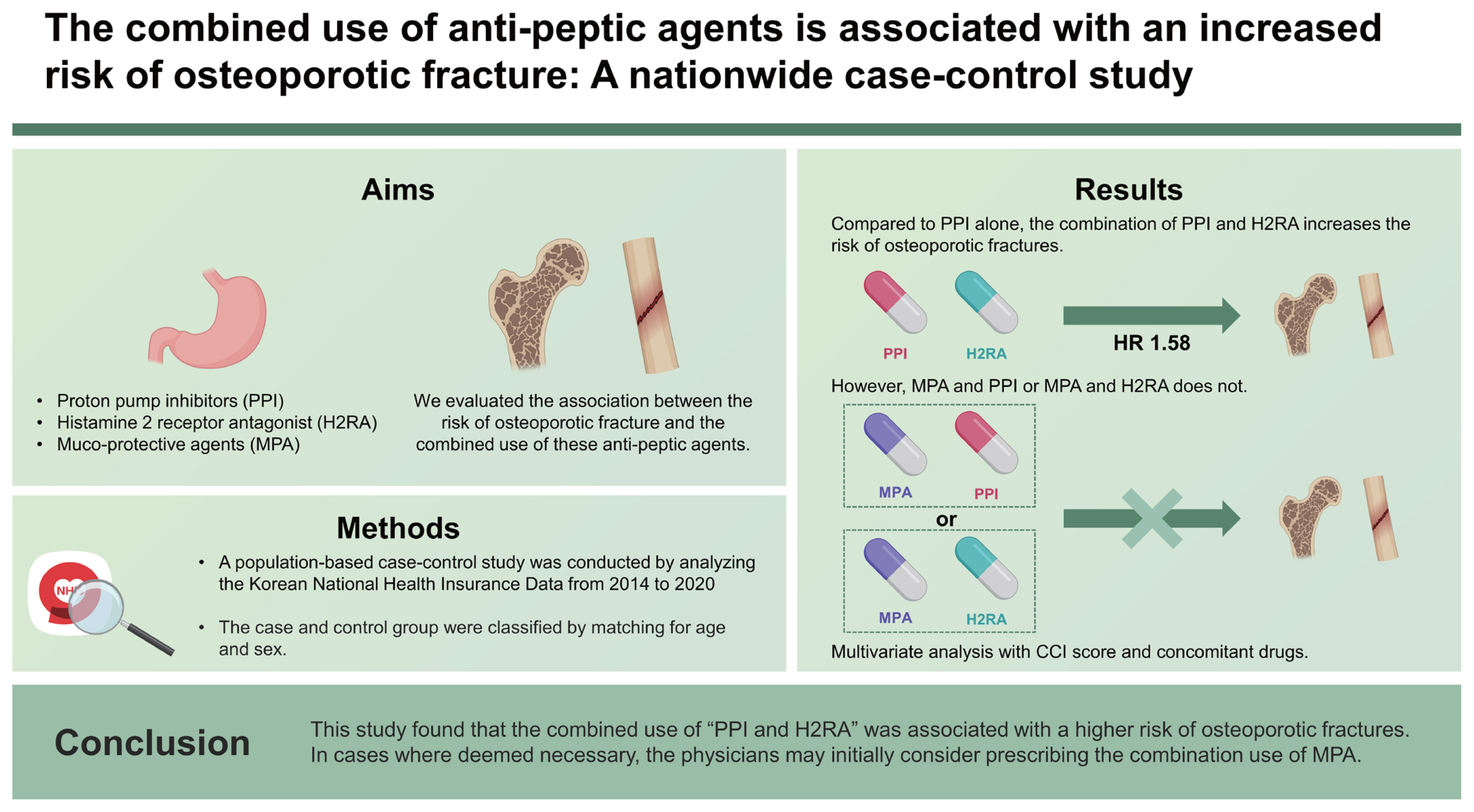
The combined use of anti-peptic agents is associated with an increased risk of osteoporotic fracture: a nationwide case-control study
Article information
Abstract
Background/Aims
Long-term use of acid suppressants such as proton pump inhibitors (PPIs) and histamine 2 receptor antagonist (H2RA) has been associated with the risk of osteoporotic fracture. Acid suppressants and muco-protective agents (MPAs) are often used together as anti-ulcer agents. We evaluated the association between the risk of osteoporotic fracture and the combined use of these anti-peptic agents.
Methods
A population-based case-control study was conducted by analyzing the Korean National Health Insurance Data from 2014 to 2020. Patients who had been prescribed anti-peptic agents, such as PPI, H2RA, or MPA, were included. Considering the incidence of osteoporotic fractures, the case group (n = 14,704) and control group (n = 58,816) were classified by 1:4 matching based on age and sex.
Results
The use of all types of anti-peptic agents was associated with an increased risk of osteoporotic fractures (PPI: hazard ratio [HR], 1.31; H2RA: HR, 1.44; and MPA: HR, 1.33; all p < 0.001). Compared to PPI alone, the combined use of “PPI and H2RA” (HR, 1.58; p = 0.010) as well as “PPI, H2RA, and MPA” (HR, 1.71; p = 0.001) was associated with an increased risk of osteoporotic fracture. However, compared with PPI alone, “MPA and PPI or H2RA” was not associated with an increased risk of osteoporotic fracture.
Conclusions
This study found that the combined use of “PPI and H2RA” was associated with a higher risk of osteoporotic fractures. In cases where deemed necessary, the physicians may initially consider prescribing the combination use of MPA.
INTRODUCTION
Polypharmacy is common in older patients with multiple comorbidities, increasing the likelihood of duplicate prescriptions for drugs with similar effects and raising the risk of drug-induced side effects [1,2]. In particular, many patients are prescribed anti-peptic agents such as proton pump inhibitors (PPIs), histamine 2 receptor antagonists (H2RAs), and muco-protective agents (MPA) for an extended duration to treat and prevent acid-related or drug-induced gastroenteropathy such as peptic ulcer disease, gastroesophageal reflux disease, and functional dyspepsia [3–8].
Because acid-related or drug-induced gastroenteropathies are a common and recurrent disease, long-term and repeated use of anti-peptic agents may be necessary to manage its symptoms [9,10]. Although there is some controversy, there have been concerns about the potential side effects associated with its long-term use, especially of PPI [3]. Long-term PPI use may increase the risk of osteoporotic fractures, with a higher risk than with H2RAs in both Eastern and Western populations [11–13].
Additionally, MPA is commonly used in combination with PPI and H2RA to treat acid-related diseases. These combination uses of anti-peptic agents may have complex effects on bone metabolism [14]. However, there are limited studies on the relationship between the combined use of anti- peptic agents including MPA and the risk of osteoporotic fractures. To address this gap, we conducted a case-control study using a nationwide cohort database to investigate the association between various uses, including possible combination use, of different anti-peptic agents and the risk of osteoporotic fracture.
METHODS
Data source
We analyzed the Korean nationwide cohort database of the National Health Insurance Service (NHIS) from 2014 to 2020. A cohort database was requested, and data were provided after the NHIS reviewed the data provision. This cohort database includes information related to the medical usage of the sample population for a 6-year period from 2014 to 2020. Korean NHIS is an insurance system that requires all citizens to subscribe to health insurance. The NHIS collects the health data of all people enrolled in the National Health Insurance and uses it for various research and policy decisions to improve national health. Information related to disease codes and drug use was confirmed using a nationwide cohort database. All methods were performed in accordance with relevant guidelines and regulations. The requirement of informed consent was waived by the appropriate board. The study protocol was approved by the Institutional Review Board (IRB) of the Dongguk University Ilsan Hospital (IRB no. DUIH 2020-05-010-002). To comply with the privacy rules of health insurance transfers and other privacy laws, all personal identification numbers were encrypted by converting them into scrambled numbers before data processing. Therefore, the use of anonymized information exempted this study from the need for written informed consent.
Study population
Patients who had been prescribed anti-peptic agents, such as PPI, H2RA, or MPA, for more than 2 days between 2014 and 2020 were selected from the NHIS database. The cohort entry date was defined as the date of the first prescription of anti-peptic agents during the study period from 2014 to 2020. The end point date was defined as the time of osteoporotic fracture occurrence in the study group and the end date of the NHIS claims data in the control group. Upon cohort entry, all patients were required to be at least 40 years old and have at least 1 year of prior medical information. Patients under the age of 50, those diagnosed with malignancy, acquired immunodeficiency syndrome, osteoporosis and fracture, and those prescribed anti-peptic agents within 1 year prior to the entry date were excluded.
The finally enrolled patients were divided into case and control groups based on the occurrence of osteoporotic fractures. In the case group, osteoporotic fractures occurred among anti-peptic agent users. Osteoporotic fractures were confirmed according to International Classification of Diseases 10th Revision (ICD-10) codes included in the medical claims data of the NHIS. This included patients with a diagnosis code of osteoporotic fracture (M80) and those with fracture codes for specific locations (thoracic vertebrae [S220–S221], lumbar vertebra [S320, S327], spine [M484, M485], proximal humerus [S422, S423], femur [S720, S721], and distal radius [S525, 526]), following treatment and diagnosis of osteoporosis (M81, M82) [15]. The control group was comprised of anti-peptic agent users who did not experience osteoporotic fractures. Case-control matching was performed between both groups using age and sex as variables.
Drug exposure and covariates
We confirmed the prescription and duration of PPI, H2RA, and MPA using the drug code (main ingredient code) in the NHIS claims database. The PPIs included rabeprazole, pantoprazole, S-pantoprazole, lansoprazole, dexlansoprazole, omeprazole, esomeprazole, and ilaprazole. The H2RAs included roxatidine, nizatidine, lafutidine, famotidine, cimetidine, and ranitidine. The MPAs included eupatilin, irsogladine, ecabet sodium, sodium alginate, misoprostol, rebamipide, teprenone, and troxipide. During the study period, the groups of anti-peptic agents were classified into groups PPI alone; H2RA alone; MPA alone; PPI and MPA; H2RA and MPA; PPI and H2RA; and PPI, H2RA, and MPA based on drug exposure. In each group, anti-peptic agents could be either used simultaneously or independently.
We also identified concomitant drugs that may affect osteoporotic fractures such as bisphosphonates, glucocorticoids, anticonvulsants, hormone replacement therapy, warfarin, heparin, antacids, selective serotonin reuptake inhibitors, benzodiazepines, and tricyclic antidepressants. Concomitant diseases such as chronic obstructive pulmonary disease (COPD), testicular dysfunction, hypothalamic dysfunction, hyperthyroidism, hyperparathyroidism, Cushing’s syndrome, hyperprolactinemia, vitamin D deficiency, idiopathic hypercalciuria, diabetes, anorexia nervosa, systemic lupus erythematosus, hypertension, intestinal absorption disorder, inflammatory bowel disease, chronic kidney disease, and secondary amenorrhea were also identified. The Charlson Comorbidity Index (CCI) score was confirmed by considering these diseases and comorbidities [16].
Study outcomes and statistical analysis
The primary outcome was to confirm the use of anti-peptic agents and the risk of osteoporotic fracture during the study period. The secondary outcomes were to determine the risk of osteoporotic fracture according to the type of anti-peptic agent used and duration of use. Categorical variables, such as sex, types of health insurance, presence of comorbidities, and presence of concomitant medications, are presented as frequencies and percentages, while continuous variables, such as age and CCI, are presented as mean and standard deviation. Conditional logistic regression analysis was performed to determine the association between the use of anti-peptic agents and the incidence of osteoporotic fractures, and the results are presented as hazard ratio (HR) and 95% confidence interval (CI). If any diseases or drugs known to be associated with osteoporotic fractures were identified during the study period, they were considered major risk factors. Logistic regression analysis was conducted for each factor to determine its statistical significance in predicting the risk of osteoporotic fractures. A significance level of p < 0.05 was used for each factor included as a covariate in the regression model. Statistical analysis was performed on all data using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA), and statistical significance was tested with a significance probability of 0.05 and a two-tailed test.
RESULTS
Baseline characteristics
During the study period, 4,950,669 patients were prescribed anti-peptic agents for more than 2 days. After excluding the patients who met the exclusion criteria, 413,404 patients remained in the study. Osteoporotic fractures were confirmed in 14,704 patients (case group) and not confirmed in 398,700 patients (control group). A 1:4 matching was performed for the case and control groups, with age and sex as matching variables; therefore, 14,704 and 58,816 patients were assigned to the case and control groups, respectively (Fig. 1).
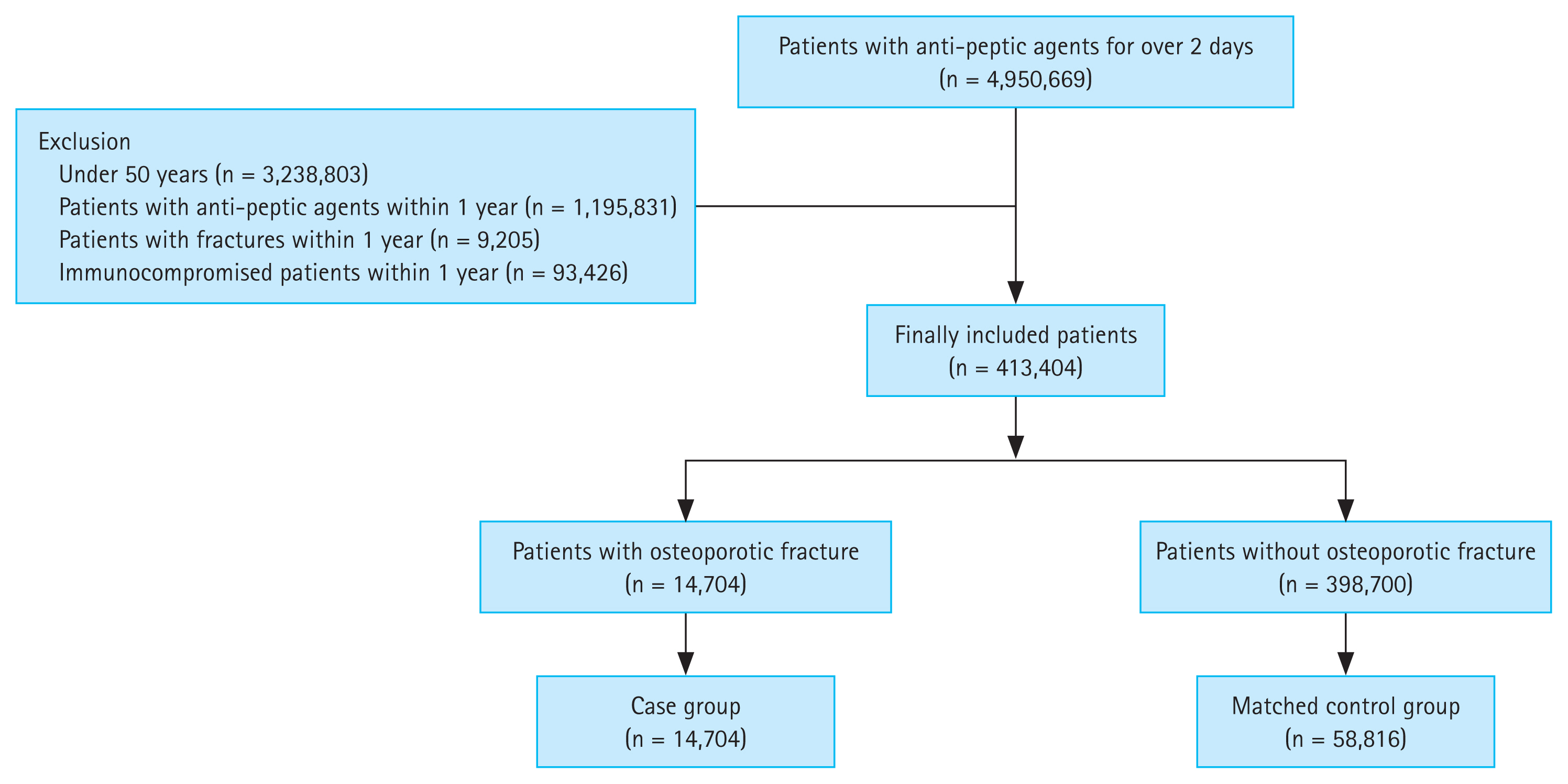
Study protocol. The case and control groups were subjected to 1:4 matching based on age and sex as variables.
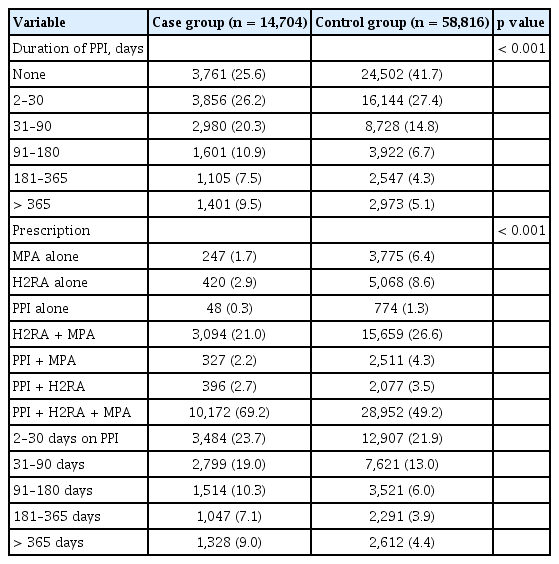
The proportion of women was 75.3% and 75.1% in the case and control groups, respectively. The mean ages of the case and control groups were 69.88 and 69.90 years, respectively, and they were appropriately matched by age. Both the case and control groups had high rates of concomitant diseases, including hypertension, diabetes, and COPD. However, these diseases were significantly more prevalent in the case group than in the control group (all p < 0.001). The number of concomitant drugs was also higher in the case group than in the control group; the proportion of patients who had been prescribed glucocorticoids was 84.9% in the case group and 74.7% in the control group. The proportion of patients with a CCI score ≥ 3 was significantly higher in the case group (62.6%) than it was in the control group (44.5%) (p < 0.001) (Table 1).
Use of anti-peptic agents
Regarding the duration of PPI use, there were more cases in which PPI were prescribed for ≥ 30 days in the case group than in the control group. During the study period, the proportions of patients who were prescribed PPI alone, H2RA alone, and MPA alone were 0.3%, 2.9%, and 1.7% in the case group and 1.3%, 8.6%, and 1.8% in the control group, respectively. The combined use of “PPI, H2RA, and MPA” was the most common administration method in both groups, prescribed in 69.2% in the case group and 49.2% in the control group. When comparing the method of anti-peptic agent use between the case and control groups, the ratio of combined use of “PPI, H2RA, and MPA” was found to be significantly higher only in the case group (p < 0.001). All other methods of anti-peptic agent use were more common in the control group (Table 2).
Risk of osteoporotic fracture and use of anti-ulcer agents
We conducted a multivariate analysis to investigate the association between the use of anti-peptic agent and the risk of osteoporotic fractures, while adjusting for concomitant drugs and the CCI score. All anti-peptic agents were associated with an increased risk of osteoporotic fractures. The risk of osteoporotic fracture was 1.31 times higher (95% CI, 1.24–1.38) with PPI alone (n = 822), 1.44 times higher (95% CI, 1.30–1.59) with H2RA alone (n = 5,488), and 1.33 times higher (95% CI, 1.22–1.45) with MPA alone (n = 4,022), with each of these results being statistically significant (all p < 0.001). However, in MPA group, eupatilin alone (n = 1,134) was not associated with an increased risk of osteoporotic fracture in the multivariate analysis (HR, 1.07; 95% CI, 1.22–1.45; p = 0.086). In a multivariate analysis comparing the risk of osteoporotic fracture based on the use of anti-peptic agents versus PPI alone, MPA alone (HR, 0.89; 95% CI, 0.62–1.27; p = 0.509), H2RA alone (HR, 0.85; 95% CI, 0.61–1.20; p = 0.362), “H2RA and MPA” (HR, 1.37; 95% CI, 0.99–1.90; p = 0.062), and “PPI and MPA” (HR, 1.22; 95% CI, 0.86–1.74; p = 0.267) showed no association with an increased risk of osteoporotic fractures. However, with “PPI and H2RA” (HR, 1.58; 95% CI, 1.11–2.23; p = 0.010) and “PPI, H2RA, and MPA” (HR, 1.71; 95% CI, 1.23–2.38; p = 0.001), the risk of osteoporotic fractures increased significantly compared to that of PPI alone (Table 3).

Univariate and multivariate analysis for association between the risk of osteoporotic fractures and the use of anti-peptic agents
In a multivariate analysis examining the duration of PPI use and concomitant use of anti-peptic agents, only the combined use of “PPI, H2RA, and MPA” showed a significantly higher risk of osteoporotic fracture compared to PPI alone, even among those who used PPI for < 30 days (HR, 2.25; 95% CI, 1.12–4.53; p = 0.023). When PPI was taken for < 90 days, the risk of osteoporotic fractures increased significantly even in “PPI and H2RA” compared to PPI alone (HR, 1.89; 95% CI, 1.16–3.09; p = 0.011). In “PPI and MPA”, compared to PPI alone, the risk of osteoporotic fracture did not significantly increase if the duration of use was < 6 months (HR, 1.40; 95% CI, 0.89–2.21; p = 0.143), whereas the risk of osteoporotic fractures increased if the duration of use was ≥ 6 months (Table 4). Moreover, we analyzed the risk of osteoporotic fractures over time from the initial prescription of the anti-peptic agents, and cumulative hazard over time was highest in the order of “PPI, H2RA, and MPA” followed by “PPI and H2RA” (Fig. 2).

Association between long-term use of anti-peptic agents and the risk of osteoporotic fractures. We analyzed the risk of osteoporotic fractures over time from the initial prescription of the anti-peptic agents. Cumulative hazard over time was highest in the order of “PPI, H2RA, and MPA” followed by “PPI and H2RA”. PPI, proton pump inhibitor; H2RA, histamine 2 receptor antagonist; MPA, muco-protective agent.
DISCUSSION
This study investigated the combined and proloned use of anti-peptic agents, especially the combination of “PPI and H2RA”, was associated with a higher risk of osteoporotic fractures. However, compared with PPI alone, “PPI and MPA” or “H2RA and MPA” did not increase the risk of osteoporotic fractures.
Osteoporotic fractures refer to fractures occurring with low energy forces, which would not typically cause fractures under normal circumstances [17]. Prevention of osteoporotic fractures is crucial because such fractures can result in significant physical, mental, and financial burdens on patients [18]. Older age, female sex, previous fragile fractures, low body weight, smoking, low dietary calcium intake, vitamin D deficiency, and some medications are known risk factors for osteoporotic fractures [19,20]. A meta-analysis of several studies suggested a possible association between the long-term use of PPI and an increased risk of osteoporosis (HR, 1.23; p < 0.05) and any-site fractures (HR, 1.30; p < 0.05), including hip, spine, and wrist fractures [21]. The risk of osteoporotic fractures increases even when PPIs are used together with steroids or bisphosphonates [22,23].
However, as demonstrated by the prescription patterns of anti-peptic agents in this study, the use of a single anti-peptic agent alone was infrequent (0.3–8.6%). Combinations of anti-peptic agents are more commonly used than single agents. In Korea, national health insurance and access to medical care are guaranteed; therefore, the number of outpatient visits is higher than that in other countries [24]. Furthermore, MPA can be used alone or in addition to acid suppressants such as PPI and H2RA in the treatment of nonsteroidal anti-inflammatory drug-related gastrointestinal injury, gastritis, and peptic ulcers [25,26]. Therefore, it is necessary to analyze the risk of osteoporotic fractures associated with the combined use of anti-peptic agents.
The multivariate analysis showed that, in comparison to PPI alone, the risk of osteoporotic fracture increased more in the combined use of “PPI and H2RA” (HR, 1.58; p = 0.010) and “PPI, H2RA, and MPA” (HR, 1.71; p = 0.001). Nonetheless, the risk of osteoporotic fracture was not significantly increased in “PPI and MPA” and “H2RA and MPA” when compared to PPI alone. Because PPI and H2RA reduce the gastric acid secretion, they may affect calcium and vitamin absorption and increase the fracture risk [27]. H2RA reportedly increases the risk of hip fractures when used for > 1 year (HR, 1.23; 95% CI, 1.14–1.39; p < 0.001) [11]. Unlike PPI and H2RA, MPAs (such as eupatilin) have anti-inflammatory and antioxidant effects and enhance gastric mucosal defense and mucosal healing [28]. Also, studies have reported that MPA such as eupatilin and rebamipide are associated with the inhibition of osteoclasts [29,30]. Because of the differences in the mechanisms of action, the risk of osteoporotic fracture may not be significantly increased by acid suppressants and MPA, unlike in the case of PPI and H2RA. However, the risk of osteoporotic fracture increased even with MPA alone (HR, 1.33). It is possible that these different effects on MPA and the risk of osteoporotic fractures are related to prostaglandin. Prostaglandin E2, involved in the MPA mechanism, is involved in bone resorption through RANKL activation and bone formation by stimulating osteoblastic differentiation [31]. Further clinical studies are needed to investigate the association between MPA and bone metabolism.
A key limitation of this study is its retrospective observational nature, interrupting the establishment of a causal relationship between the use of anti-peptic agents and the risk of osteoporotic fracture. Additionally, osteoporotic fractures may be attributed to several risk factors in the patient, and it is essential to consider that the use of anti-peptic agents might not be the sole contributing factor. Despite adjusting for factors such as age, sex, and concomitant medications, and the CCI scores that can affect osteoporotic fractures, other potential confounding factors that may have influenced the risk of osteoporotic fractures, such as smoking, alcohol intake, physical activity, and body mass index, were not evaluated in this study. Moreover, based on the definition of the study population, there could be instances within groups of anti-peptic agents where individuals did not take these agents simultaneously. Nonetheless, we aimed to determine whether the use of different types of anti-peptic agents, as well as their simultaneous use, is associated with the risk of osteoporotic fractures. Therefore, further research is needed to explore the association between the simultaneous use and cumulative effects of anti-peptic agents and the risk of osteoporotic fractures.
Nevertheless, the findings of this study showed that patients who took multiple types of anti-peptic agents or their combination had a significantly higher risk of osteoporotic fractures than those who did not. In addition, the risk was highest in patients who had been using multiple anti-peptic agents for a long period. Although anti-peptic agents do not cause osteoporotic fractures, physicians should carefully evaluate the benefits and risks of using these agents. If possible, physicians should prioritize treatment options involving combination of MPA, which does not increase the risk of osteoporotic fractures, over that of PPI and H2RA. Further studies are warranted to determine the exact mechanism by which these anti-peptic agents may contribute to fractures and identify strategies for minimizing the risk of fractures in patients who require long-term use of anti-peptic agents.
KEY MESSAGE
1. Compared to PPI alone, the combined uses of “PPI and H2RA” (HR, 1.58; 95% CI, 1.11–2.23; p = 0.010) and “PPI, H2RA, and MPA” (HR, 1.71; 95% CI, 1.23–2.38; p = 0.001) were each associated with an increased risk of osteoporotic fracture.
2. Compared to PPI alone, the combined uses of “MPA and PPI” and “MPA and H2RA” were not associated with an increased risk of osteoporotic fracture.
3. Physician, if feasible, should consider prescribing the combination of MPA, which does not increase the risk of osteoporotic fractures, over that of PPI and H2RA.
Acknowledgments
The authors thank Hyun Jung Ahn for technical assistance for this study.
Notes
CRedit authorship contributions
Dong Jun Oh: conceptualization, methodology, formal analysis, writing - original draft, visualization; Ji Hyung Nam: formal analysis, validation, writing - review & editing; Hyun Seok Lee: formal analysis, validation, writing - review & editing; Yeo Rae Moon: data curation, formal analysis, software; Yun Jeong Lim: conceptualization, methodology, investigation, writing - review & editing, supervision, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by grants received from DONG-A ST pharmaceutical research fund and Dongguk University research fund.