Duration of corticosteroid treatment in hospitalized COVID-19 patients, less is more
Article information
Dear Editor,
We have read with great interest the paper by Kim et al. [1] who reported improved survival and no increase in nosocomial complications with prolonged corticosteroid use in severe COVID-19 patients. Since the two groups of corticosteroid users were defined by a post-baseline feature (duration of corticosteroid use > 14 days), there is a high risk of immortalization bias, i.e., patients who died early during hospitalization are automatically classified as short-term corticosteroid users, inevitably biasing the final conclusions. Thus, we aimed to evaluate the same phenomenon in our cohort of severe and critical COVID-19 patients treated in a tertiary level institution, University hospital Dubrava, Zagreb, Croatia in period from March 2020 to June 2021. All patients were Caucasian adults. The majority of deaths occurred during the first 14 days of hospitalization (59.7% of all deaths, median time of death 12 days from admission). Therefore, out of a total of 1,558 available patients, we selected a cohort of 1,129 patients who survived for at least 14 days post-hospital admission and who were treated with corticosteroids. Corticosteroid doses were classified as low doses (below 1 mg/kg body weight, given in 54.6% patients), high doses (1–2 mg/kg body weight, given in 36.8% patients) and very high doses (above 2 mg/kg body weight, given in 8.6% patients). Most patients received dexamethasone (77.5%), followed by methylprednisolone (45.6%) and prednisone (5.4%). Patients started corticosteroids on the median first day of hospitalization. Patients were concomitantly treated with remdesivir (54.1%) and low molecular weight heparins in prophylactic (20.9%), intermediate (33.9%) and therapeutic doses (43%).
Median age of patients was 65 years, interquartile range (interquartile range [IQR] 56–72). Median Charlson comorbidity index was 3 points, IQR (1–4). There were 63.5% male and 36.5% female patients. Intrahospital mortality, mechanical ventilation (MV), venous thromboembolism (VTE), major bleeding (MB) and bacterial blood stream infection (BSI) rates in 14-days survivors were 16.8%, 20.3%, 8%, 3.2% and 10.4%, respectively. Patients with longer duration of corticosteroid treatment were significantly older (median 66 vs. 64 yr, p < 0.001), had slightly higher comorbidity burden (median Charlson comorbidity index 3 vs. 3 points, p < 0.001) and required higher intensity of oxygen supplementation at admission (median oxygen flow 10 vs. 5 L/min) but did not significantly differ regarding sex nor remdesivir exposure (p > 0.05 for both analyses).
Corticosteroid use > 14 days was significantly associated with higher mortality (29% vs. 12.9%; hazard ratio [HR], 2.64; 95% confidence interval [CI], 1.91–3.66; log-rank test p < 0.001) as shown in Figure 1A. This association persisted (HR, 1.49; 95% CI, 1.11–2.01; p = 0.007) in multivariate Cox regression model adjusted for age (p < 0.001), sex (p = 0.087), required oxygen supplementation at admission (p < 0.001), Charlson comorbidity index (p = 0.002) and remdesivir use (p = 0.552). Corticosteroid use > 14 days was also significantly associated with higher rates of MV (34.1% vs. 15.8%, chi-squared test p < 0.001), VTE (16.3% vs. 5.3%, chi-squared test p < 0.001), MB (5.8% vs. 2.3%, chisquared test p = 0.005) and BSI (17% vs. 8.2%, chi-squared test p < 0.001).
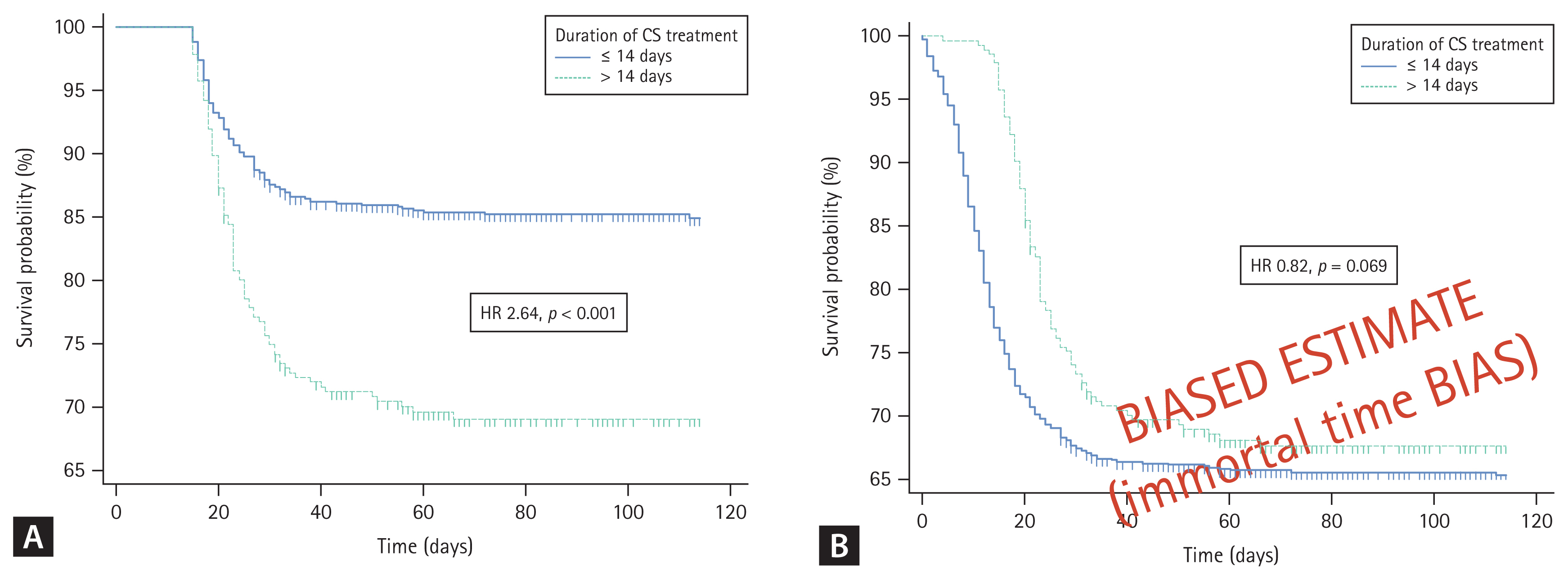
(A) Survival post 14-days after hospital admission stratified by duration of corticosteroid (CS) use (≤ 14 and > 14 days of treatment). (B) Survival post hospital admission without implementing 14-days survival time landmark.
In contrast to report by Kim et al. [1], our data suggest that prolonged corticosteroid use in severe and critical COVID-19 patients may result in higher mortality and higher rates of complications of corticosteroid treatment (VTE, MB, BSI). Different conclusions could be obtained from our dataset as well, if conditional 14-days survival time was not implemented (Fig. 1B). However, results based on a post-baseline definition of a variable that cannot be achieved if the patients died previously, are inevitably biased towards more favorable outcomes associated with having the designated feature. This is also known as the phenomenon termed immortalization or immortal time bias [2]. Thus, we would like to suggest the authors to evaluate whether associations observed in their study persist after implementing mandatory 14-day survival condition to further clarify whether prolonged corticosteroid use may indeed benefit the patients.
Data availability
Data can be obtained from the corresponding author per reasonable e-mail request.
Notes
CRedit authorship contributions
Marko Lucijanic: conceptualization, methodology, investigation, validation, writing - original draft, writing - review & editing, supervision, project administration; Ivan Papic: methodology, investigation, validation, writing - original draft, writing - review & editing; Maja Ortner Hadziabdic: conceptualization, investigation, validation, writing - original draft, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None
Ethical approval
The study was approved by the University Hospital Dubrava Review Board (Nm. 2021/1410-01). The study was conducted in accordance with the Declaration of Helsinki.